22.0: Chapter Objectives and Introduction to Carbonyl Alpha-Substitution Reactions
- Page ID
- 36420
After completing this section, you should be able to write a general mechanism for an alpha‑substitution reaction of a carbonyl compound.
Make certain that you can define, and use in context, the key terms below.
- alpha (α) position
- alpha‑substitution reaction
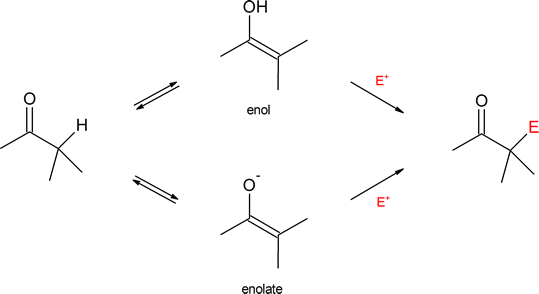
An “alpha‑substitution reaction” of a carbonyl compound is a reaction in which one of the hydrogen atoms on the carbon adjacent to the carbonyl group is substituted by some other atom or group. Attack by the electrophile (E+) can occur on the enol or enolate intermediate.
There are four common types of reactions involving compounds containing a carbonyl bond. The first two, nucleophilic addition and nucleophilic acyl substitution, have been discussed in previous chapters.
Nucleophilic addition occurs due to the electrophilic nature of the carbonyl carbon. After addition of a nucleophile, the carbonyl becomes a tetrahedral alkoxide intermediate which is usually protonated to become an -OH group.
Nucleophilic Addition to a Carbonyl
Nucleophilic acyl substitution is similar in that a tetrahedral alkoxide intermediate is formed after nucleophilic addition to the carbonyl. However, subsequent removal of the leaving group allows for the C=O (carbonyl) bond to reform. Overall, there is a substitution of the leaving group with the incoming nucleophile.
Nucleophilic Acyl Substitution Involving a Carbonyl
Reactions at The Alpha Carbon
The remaining common carbonyl reaction types are α-substitutions and carbonyl condensations. Both utilize the special properties of carbons directly adjacent to carbonyls which are called α-carbons. These reactions, which can be regarded as the backbone of much synthetic organic chemistry, usually result in the replacement of a hydrogen attached to an α-carbon with some type of electrophile. These reactions involve two new nucleophilic species called the enol and the enolate.
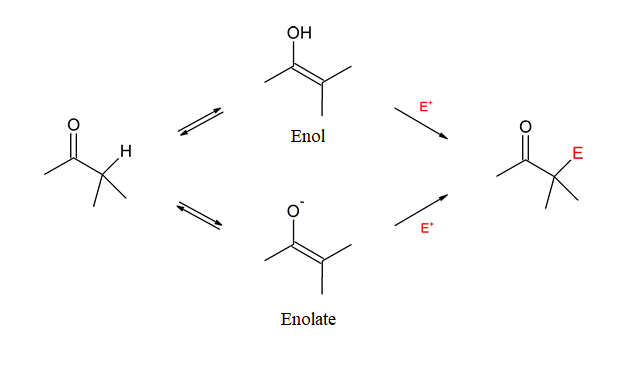
This chapter will focus on α-substitutions reactions. Although there are many carbonyl containing functional groups, the initial investigation in this chapter will focus on α-substitutions reactions using aldehydes and ketones. Important examples considered in this chapter include α-halogenation and α-alkylation.

