21.9: Polyamides and Polyesters - Step-Growth Polymers
- Page ID
- 36406
After completing this section, you should be able to
- write an equation to represent the formation of nylon 66 from adipic acid and hexamethylenediamine.
- write an equation to represent the formation of nylon 6 from caprolactam.
- predict the structure of the step‑growth polymer formed from the reaction of two given monomers.
- identify the monomers needed to prepare a step‑growth polymer of a given structure.
- write an equation to describe the formation of a polyester, such as poly(ethylene terephthalate), from a given diol and diester.
- write an equation to describe the formation of a polycarbonate from a carbonate and a bisphenol.
Make certain that you can define, and use in context, the key terms below.
- chain‑growth polymer
- polyamide
- polyester
- step‑growth polymer
You may wish to review chain‑growth polymerization in Section 8.10 and Section 14.6. In this section we discuss another important class of polymerization kknow as step‑growth polymerization.
As you can see from the equation given in the discussion on forming condensation polymers, Dacron is formed from two monomers, one of which possesses two carboxylate groups and the other two hydroxyl groups.
It is also possible to form a polyester using two monomers that each possesse one carboxyl group and one hydroxyl group. An example is the polymer formed between lactic acid and glycolic acid. This polymer has been used to produce absorbable staples that provide surgeons with a convenient method of closing incisions made during operations on the bladder or intestines, or during hysterectomies. Two advantages of this polyester in such applications are, first, that it begins to hydrolyze in the body after six to eight weeks, and then, that the products of the hydrolysis are both compounds normally present in the body.
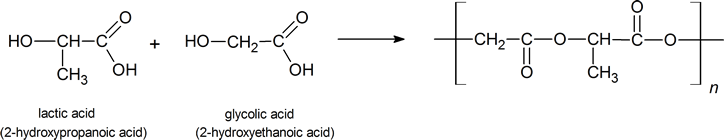
Step-Growth Polymerizations
Step-growth and chain-growth are two broad classes of polymerization methods. Sections 8.11 and 14.7 discussed how chain-growth polymers usually start with a single alkene containing monomer, which is unreactive until an initiator is added. The initiator converts one monomer molecule into a reactive intermediate (radical, cation, anion) which is capable of reacting with another monomer forming a C-C bond and creating a new reactive intermediate. This reactive intermediate reacts with another monomer and the process is repeated as a chain reaction to form a polymer.
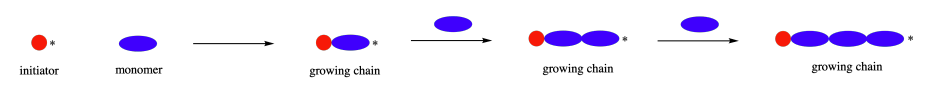
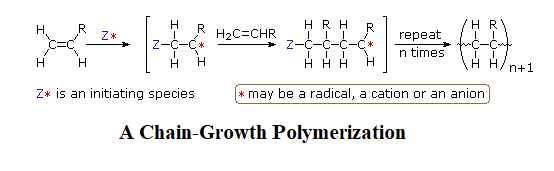
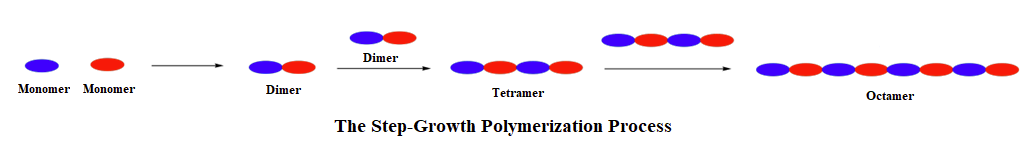
The process of step-growth polymerizations are fundamentally different than in chain-growth. In step-growth polymerizations, monomers are generally linked by a carbon-heteroatom bond (C-O & C-N) formed in non-sequential steps. Often, the reactions used to link these monomers include multiple nucleophilic acyl substitutions. Step-growth polymerizations usually use two different monomers, neither of which would undergo polymerization on its own. The two monomers are multifuntional and complementary to each other, such that each provides the other with a reactive partner. In this section, we will be focusing on monomers which are difunctional, meaning they contain two of the same reactive functional group. A step-growth polymerization starts with two complementary functional groups on different monomers reacting to form a dimer. Because both monomers were difunctional, each retains a reactive group and can react with additional complementary monomers.
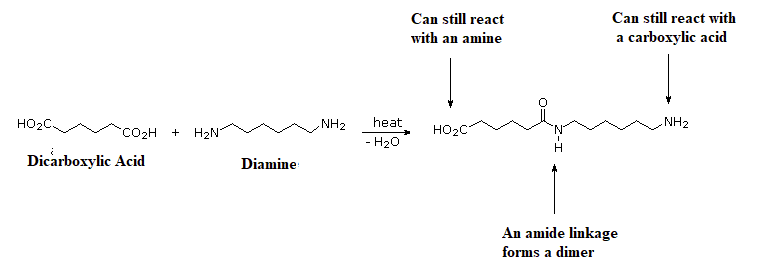
In fact the difuctionality of the monomers, allows step-growth polymers to grow in two directions at once. First, two complementary monomers react with each other to form a dimer. Assuming that monomers react at roughly similar rates, when one end of the dimer reacts again it will likely find another dimer and form a tetramer. Then when the tetramer goes to react again it will most likely find another tetramer and form an octamer. This process is repeated allowing the polymer to grow in two directions at the same time.
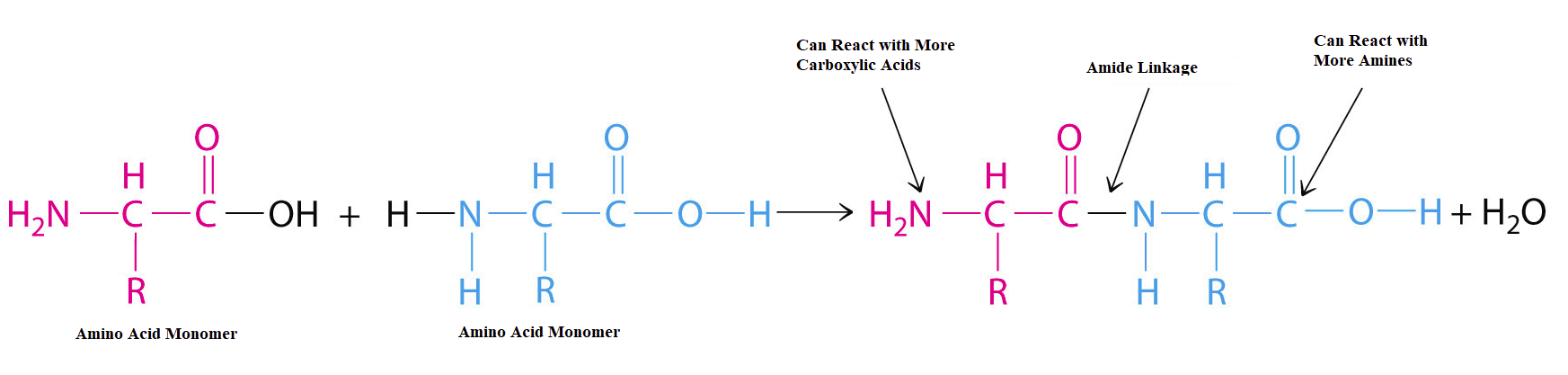
Virtually all fibers are made from some form of polymer. In particular, silk and wool are composed of a naturally occurring protein polymer. The monomers of proteins are called amino acid residues. These residues are connected by amide linkages which are also called peptide bonds. Many of the early efforts of polymer chemistry were to artificially create fibers which mimicked the properties of silk and wool.
Polyamides
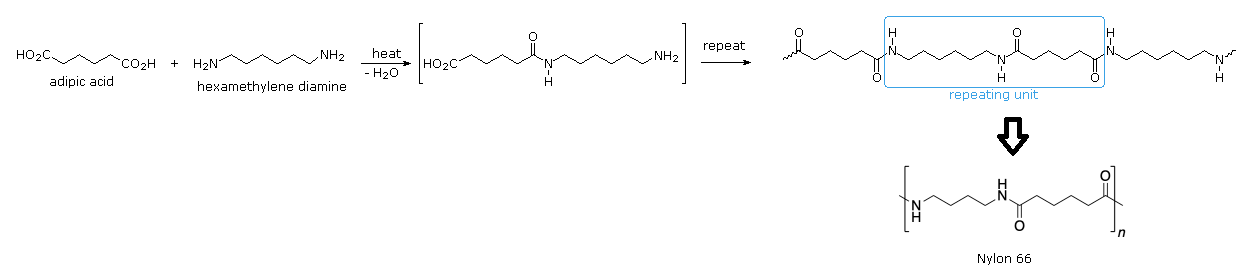
The first fully synthetic polymer fiber, nylon-6,6, was produced in 1938 by the company DuPont. The lead chemist of DuPont's work was Wallace H. Carothers, who reasoned that the properties of silk could be mimicked by constructing a polymer chain created with repeating amide bonds, just like the proteins in silk. Nylon-6,6 was created by first reacting 1,6-hexanedioic acid (adipic acid) and 1,6-hexanediamine to give a salt which was then heated creating multiple amide bonds through nucleophilic acyl substitution. The product of this particular reaction is a polyamide called nylon-6,6. The numbers of the name indicate how many carbons are contained in each monomer. The first “6” stands for the number of carbons in the diamine monomer while the second number indicated the number of carbons in the dicarboxylic acid. Simply by varying the number of carbons in each monomer, a wide variety of nylon polymers can be made.
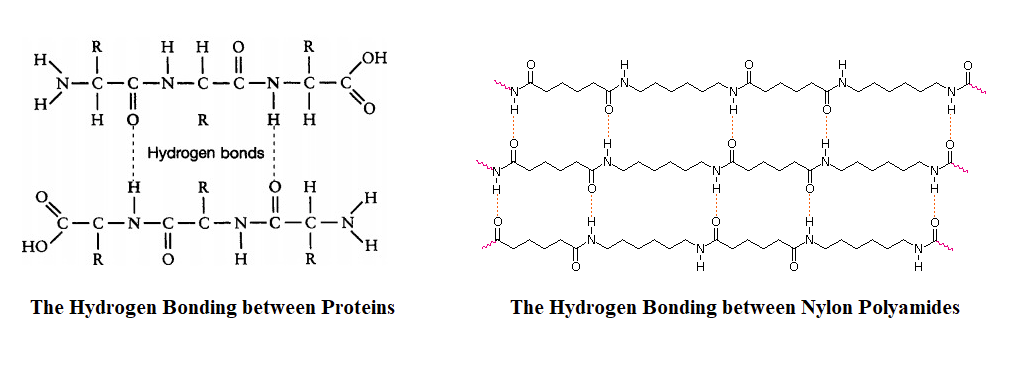
 Nylons are among the most widely used synthetic fibers—for example, they are used in ropes, sails, carpets, clothing, tires, brushes, and parachutes. Known for their high strength and abrasion resistance, nylons can be molded into blocks for use in electrical equipment, gears, bearings, and valves. The strength of nylon fibers comes, in part, from their ability to form strong hydrogen bonding intermolecular forces with each other in much the same fashion as proteins.
Nylons are among the most widely used synthetic fibers—for example, they are used in ropes, sails, carpets, clothing, tires, brushes, and parachutes. Known for their high strength and abrasion resistance, nylons can be molded into blocks for use in electrical equipment, gears, bearings, and valves. The strength of nylon fibers comes, in part, from their ability to form strong hydrogen bonding intermolecular forces with each other in much the same fashion as proteins.
Polyesters
Esterfication, via nucleophilic acyl substitutions, can also be used to form the primary linkages in step-growth polymers. A polyester is typically produced when a dicarboxylic acid and a diol are reacted together. After the initial reaction, the ester product contains a free (unreacted) carboxyl group at one end and a free alcohol group at the other. Further esterification using a step-growth polymerization, produces a polyester. The most important polyester, polyethylene terephthalate (PET), is made from the reaction of 1,4-benzenedicarboxylic (terephthalic acid) and 1,2-ethanediol (ethylene glycol) monomers.
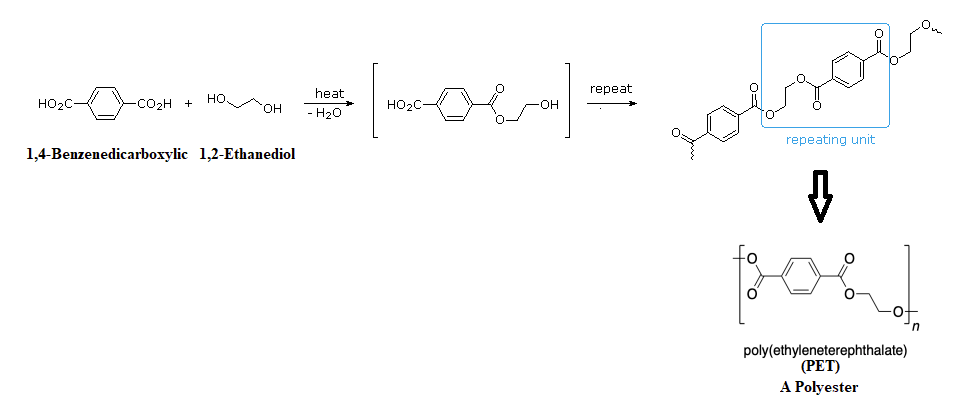
Polyester molecules make excellent fibers and are used in many fabrics. A knitted polyester tube, which is biologically inert, can be used in surgery to repair or replace diseased sections of blood vessels. PET is used to make bottles for soda and other beverages. It is also formed into films called Mylar. When magnetically coated, Mylar tape is used in audio- and videocassettes.
Polycarbonates
Beyond carboxylic acid derivatives, virtually any reaction which involves reactive species on two different molecules can be used to perform a step-growth polymerization. A variation involves using a monomer containing a carbonate functional group. A carbonate acts like a double ester and can undergo a type of double transesterification reaction with two alcohols to form a new carbonate containing compound.
In effect, a carbonate is difunctional and can be reacted with a diol to form polymers containing repeated carbonate groups in their structure called polycarbonates. An example of a polycarbonate is the polymer, Lexan, which is created when diphenyl carbonate and bisphenol A (a diol) are reacted together.
Bisphenol (BPA), primarily used to make polycarbonate, is one of the highest-volume chemicals produced in the world, with over six billion pounds made each year. Because polycarbonate is used to make plastic bottles, the lining for food cans, and the lining for beverage cans there has been much concern about trace amounts of BPA leaching from the containers and being ingested. A study conducted in 2003 and 2004 by the Center for Disease Control and Prevention found trace amounts BPA in the tissues of 93% of people in the United States. Consequently, this has led to many beverage companies switching to non-polycarbonate polymers.
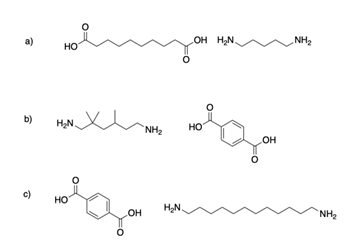
When drawing the polymer product of a step-growth polymerization, it is important to first identify the reaction which forms the repetitive linkage between monomers. In this case, the reaction is anucleophilic acyl substitution btween a carboxylic acid and an amine to form an amide. In total, the bracket structure of a polymer shows two versions of the linkage formation. The first is clearly shows connecting the monomers to form the dimer making up the repeating unit in the polymer. The second version involves bonds going out of the bracket and represents how the linkage repeats throughout the polymer. 1) The connect two monomers to make a dimer using the indicated reaction. 2) Beak the same bonds as before ecepptt the bonds for the final connection will be shown going away from the dimer. 3) Place brackets around the dimer and put an italicized lower case "n" in the lower right.
Solution
Exercises
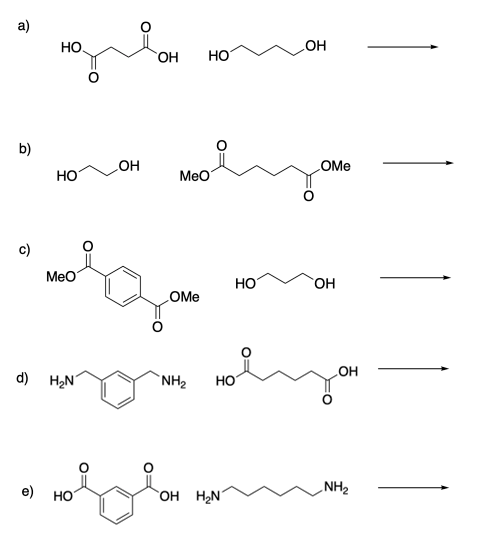
1)
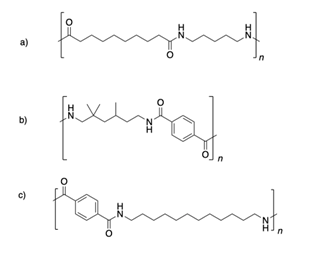
2)
Solutions
1)
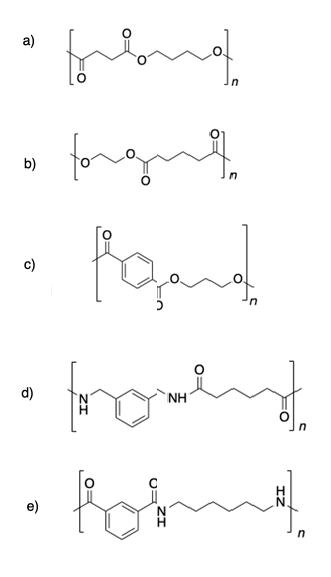
2)