3.8: 3.8 Alcohols - Classification and Nomenclature
- Page ID
- 111231
learning objectives
- classify alcohols as primary, secondary, or tertiary
- name alkanes using IUPAC (systematic) and selected common name nomenclature
- draw the structure of alkanes from IUPAC (systematic) and selected common namesAlcohol Classification
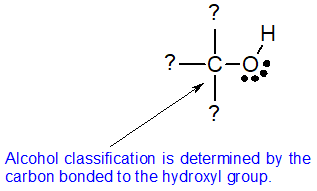
Alcohol Classification
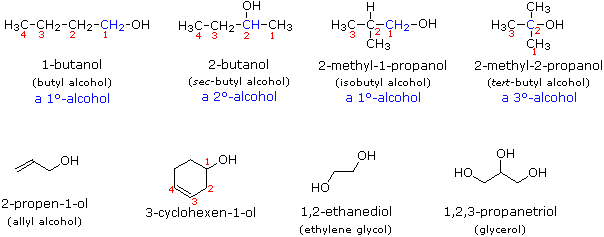
Alcohols may can be classified as primary, 1º, secondary, 2º & tertiary, 3º, in the same manner as alkyl halides. This terminology refers to alkyl substitution of the carbon atom bearing the hydroxyl group (colored blue in the illustration). This classification system is based on the neutral bonding pattern for oxygen. The structure of alcohols requires one of the oxygen bonds to form with hydrogen and the other oxygen bond to form with carbon. All oxygen atoms of alcohols look the same. To distinguish between alcohol classifications, we must look at the carbon atom bonded to the hydroxyl group. The bonding pattern of this carbon atom determines the classification of the alcohol.
Primary alcohols
In a primary (1°) alcohol, the carbon which carries the -OH group is only attached to one alkyl group. Some examples of primary alcohols include:
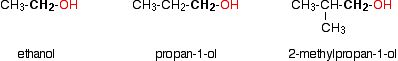
Notice that it doesn't matter how complicated the attached alkyl group is. In each case there is only one linkage to an alkyl group from the CH2 group holding the -OH group. There is an exception to this. Methanol, CH3OH, is counted as a primary alcohol even though there are no alkyl groups attached to the carbon with the -OH group on it.
Secondary alcohols
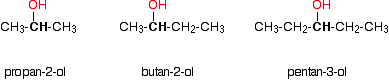
In a secondary (2°) alcohol, the carbon with the -OH group attached is joined directly to two alkyl groups, which may be the same or different. Examples:
Tertiary alcohols
In a tertiary (3°) alcohol, the carbon atom holding the -OH group is attached directly to three alkyl groups, which may be any combination of same or different. Examples:
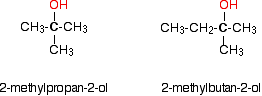
Nomenclature - IUPAC Introduction & Common Names
Alcohols are designated by an ol suffix if they are the highest priority functinal group. For example, ethanol contains a hydroxyl group to form an alcohol as shown in the follwowing condensed formula: CH3CH2OH. Note that a locator number is not needed on a two-carbon chain, but on longer chains the location of the hydroxyl group determines chain numbering and must be specified in the name. For example: CH3CH(OH)CH2CH2CH3 is 2-pentanol or pentan-2-ol.
Other examples of IUPAC nomenclature are shown below, together with the common names often used for some of the simpler compounds. For the mono-functional alcohols, this common system consists of naming the alkyl group followed by the word alcohol.
Many functional groups have a characteristic suffix designator, and only one such suffix (other than "ene" and "yne") may be used in a name. When the hydroxyl functional group is present together with a function of higher nomenclature priority, it must be cited and located by the prefix hydroxy and an appropriate number. For example, lactic acid has the IUPAC name 2-hydroxypropanoic acid.
The terms "vicinal" and "geminal" can be applied to any two functional groups that are part of the same compound. Typically, these terms are first encountered with alcohols. Vicinal is used to describe the structure of a compound in which the two groups are bonded to neighboring carbons. Geminal is used when both functional groups are bonded to the same carbon. In Latin, "gemini" means twins. In the same way that twins are connected to the same mother, geminal groups are bonded to the same carbon. Similarly, the vicinal groups are in vicinity of each other.
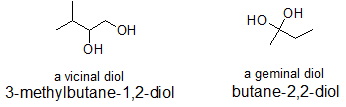
Naming Alcohols
- Find the longest chain containing the hydroxy group (OH). If there is a chain with more carbons than the one containing the OH group it will be named as a subsitutent.
- Place the OH on the lowest possible number for the chain. With the exception of carbonyl groups such as ketones and aldehydes, the alcohol or hydroxy groups have first priority for naming.
- When naming a cyclic structure, the -OH is assumed to be on the first carbon unless the carbonyl group is present, in which case the later will get priority at the first carbon.
- When multiple -OH groups are on the cyclic structure, number the carbons on which the -OH groups reside.
- Remove the final e from the parent alkane chain and add -ol. When multiple alcohols are present use di, tri, et.c before the ol, after the parent name. ex. 2,3-hexandiol. If a carbonyl group is present, the -OH group is named with the prefix "hydroxy," with the carbonyl group attached to the parent chain name so that it ends with -al or -one.
Examples
Ethane: CH3CH3 ----->Ethanol: 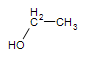 (the alcohol found in beer, wine and other consumed sprits)
(the alcohol found in beer, wine and other consumed sprits)
Secondary alcohol: 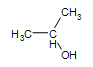 2-propanol
2-propanol
Other functional groups on an alcohol: 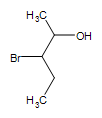 3-bromo-2-pentanol
3-bromo-2-pentanol
Cyclic alcohol (two -OH groups): 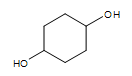 cyclohexan-1,4-diol
cyclohexan-1,4-diol
Other functional group on the cyclic structure: 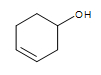 3-hexeneol (the alkene is in bold and indicated by numbering the carbon closest to the alcohol)
3-hexeneol (the alkene is in bold and indicated by numbering the carbon closest to the alcohol)
A complex alcohol: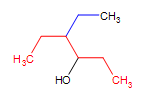 4-ethyl-3hexanol (the parent chain is in red and the substituent is in blue)
4-ethyl-3hexanol (the parent chain is in red and the substituent is in blue)
Exercise
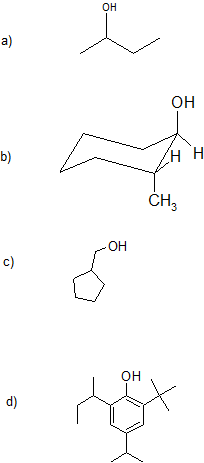
1. Give the IUPAC (Systematic) name for each compound. For parts (a)-(c), classify the alcohols as primary, secondary, or tertiary.
Part (d) is a challenge question and sneak preview of coming attractions.
2. Draw the bond line structures, condensed strucutre, and name all the alcohols with the molecular formula C3H9O.
3. Oleic acid, a commonly occurring fatty acid in vegetable oils has the structure below.
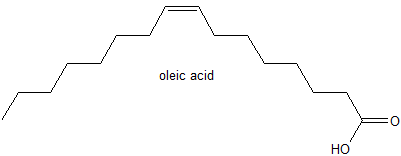
a) Describe the stereochemistry of oleic acid - cis or trans?
b) Write the condensed formula for oleic acid.
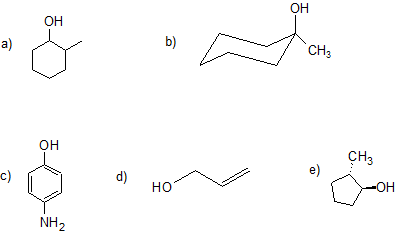
4. Give the IUPAC name for each compound. For parts (a), (b), (d), and (e) classify the alcohol as primary, secondary, tertiary, or allylic. Part (c) is also a challenge question.
- Answer
-
1. a) 2-butanol or butan-2-ol; secondary
b) 2-methylcyclohexan-1-ol or 2-methyl-1-cyclohexanol; secondary
c) cyclopentylmethanol (alcohol is higher priority over carbon chain or ring size); primary
d) 2-(butan2-yl)-6-tert-butyl-4-(propan-2-yl)phenol or 2-sec-butyl-6-t-butyl-4-isopropylphenol (some common names are recognized by IUPAC)
2.

3. a) cis alkene
b) CH3(CH2)6CHCH(CH2)6CO2H
4. a) 2-methyl-1-cyclohexanol or 2-methyl-cyclohexan-1-ol (no stereochemistry communicated even though it is possible); secondary
b) 1-methyl-1-cyclohexanol or 1-methyl-cyclohexan-1-ol (no steereochemicstry because both groups are bonded to the same carbon); tertiary
c) 4-nitrophenol
d) 2-propen-1-ol (alcohols have higher priority than alkenes so determine numbering and suffix); allylic
e) (1S, 2S)-2-methylcyclopentan-1-ol (For students who have learned about chirality); secondary
There are two equivalent answers for students who have not yet learned about chirlatiy:
trans-1-methyl-2-cyclpentanol or trans-1-methylcyclopentan-1-ol
Contributors
- William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

