3.5: Haloalkane - Classification and Nomenclature
- Page ID
- 111069
learning objectives
- classify alkyl halides as primary, secondary, or tertiary
- name alkyl halides using IUPAC (systematic) and selected common name nomenclature
- draw the structure of alkyl halides from IUPAC (systematic) and selected common names
The haloalkanes, also known as alkyl halides, are a group of chemical compounds comprised of an alkane with one or more hydrogens replaced by a halogen atom (fluorine, chlorine, bromine, or iodine). There is a fairly large distinction between the structural and physical properties of haloalkanes and the structural and physical properties of alkanes. As mentioned above, the structural differences are due to the replacement of one or more hydrogens with a halogen atom. The differences in physical properties are a result of factors such as electronegativity, bond length, bond strength, and molecular size. A few representative alklyl halides are shown below.
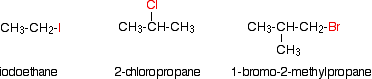
Alkyl halides are a versatile and useful functional group for multi-step organic synthesis. The reactivity of the alkyl halides can be predicted using their structural classifications. To communicate the three different structures, the terms primary, secondary, and tertiary are used. The classification is determined by the number of carbons bonded to the carbon bearing the halide. This classification strategy is analogous to the one used for alcohols and is explained in further detail below.
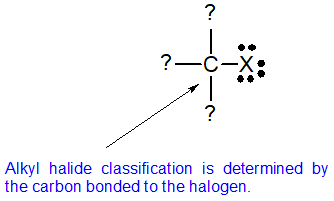
Classification of Alkyl Halides
Functional group classifications are based on the bonding patterns of the atoms involved. There is only one neutral bonding pattern for halogens (three lone pairs and a single bond) so the halogens cannot be used to determine their classification. To determine the classification of alkyl halides, the bonding pattern of the carbon bonded to the halogen is used as shown in the diagram below.
Primary alkyl halides
In a primary (1°) halogenoalkane, the carbon which carries the halogen atom is only attached to one other alkyl group.Some examples of primary alkyl halides include:
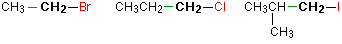
Notice that it doesn't matter how complicated the attached alkyl group is. In each case there is only one linkage to an alkyl group from the CH2 group holding the halogen. There is an exception to this: CH3Br and the other methyl halides are often counted as primary alkyl halides even though there are no alkyl groups attached to the carbon with the halogen on it.
Secondary alkyl halides
In a secondary (2°) halogenoalkane, the carbon with the halogen attached is joined directly to two other alkyl groups, which may be the same or different. Examples:
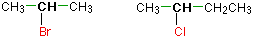
Tertiary alkyl halides
In a tertiary (3°) halogenoalkane, the carbon atom holding the halogen is attached directly to three alkyl groups, which may be any combination of same or different. Examples:
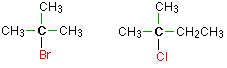
IUPAC and Common Nomenclature
The replacement of only one hydrogen atom gives an alkyl halide (or haloalkane) so the nomenclature system is closely related to the system for alkanes. The common names of alkyl halides consist of two parts: the name of the alkyl group plus the stem of the name of the halogen, with the ending -ide.
Common Name Format: alkyl name + halide name
The IUPAC system uses the name of the parent alkane with a prefix indicating the halogen substituents, preceded by number indicating the substituent’s location. The prefixes are fluoro-, chloro-, bromo-, and iodo-. Thus CH3CH2Cl has the common name ethyl chloride and the IUPAC name chloroethane. For simple halo alkanes, the IUPAC name includes the three parts shown below.
IUPAC Name Format: locator # + halo prefix + parent alkane
Alkyl halides with simple alkyl groups (one to four carbon atoms) are often called by common names. Those with a larger number of carbon atoms are usually given IUPAC names.
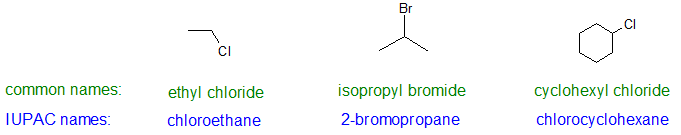
Example
Give the common and IUPAC names for each compound.
- CH3CH2CH2Br
- (CH3)2CHCl
Solution
- The alkyl group (CH3CH2CH2–) is a propyl group, and the halogen is bromine (Br). The common name is therefore propyl bromide. For the IUPAC name, the prefix for bromine (bromo) is combined with the name for a three-carbon chain (propane), preceded by a number identifying the carbon atom to which the Br atom is attached, so the IUPAC name is 1-bromopropane.
- The alkyl group [(CH3)2CH–] has three carbon atoms, with a chlorine (Cl) atom attached to the middle carbon atom. The alkyl group is therefore isopropyl, and the common name of the compound is isopropyl chloride. For the IUPAC name, the Cl atom (prefix chloro-) attached to the middle (second) carbon atom of a propane chain results in 2-chloropropane.
Exercise
Give common and IUPAC names for each compound.
- CH3CH2I
Exercise
Give the IUPAC name for each compound.
3.
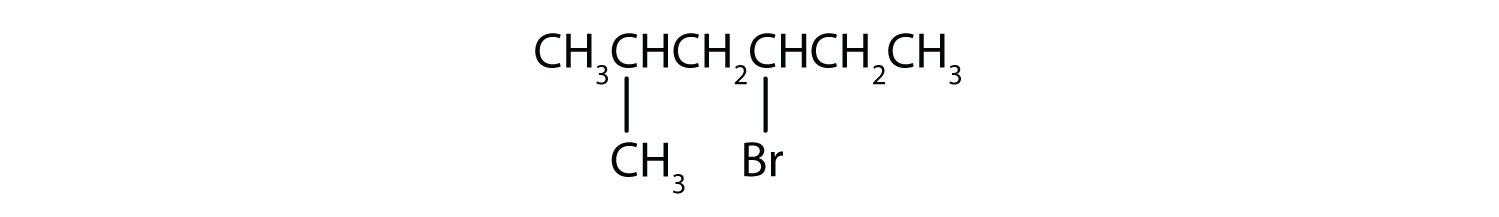
Solution
3. The parent alkane has five carbon atoms in the longest continuous chain; it is pentane. A bromo (Br) group is attached to the second carbon atom of the chain. The IUPAC name is 2-bromopentane.
4. The parent alkane is hexane. Methyl (CH3) and bromo (Br) groups are attached to the second and fourth carbon atoms, respectively. Listing the substituents in alphabetical order gives the name 4-bromo-2-methylhexane.
Exercise
Give the IUPAC name for each compound.
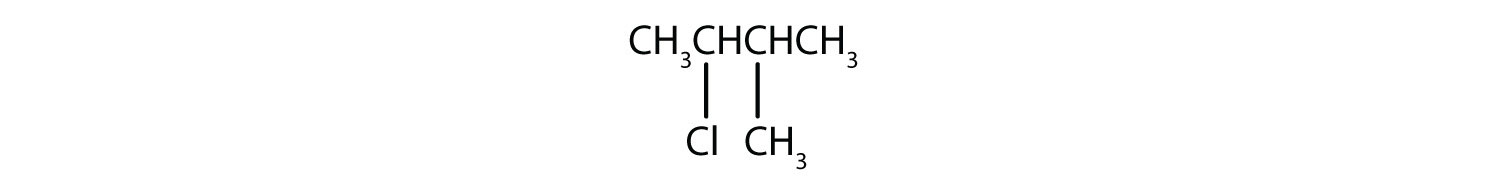
5.
6.
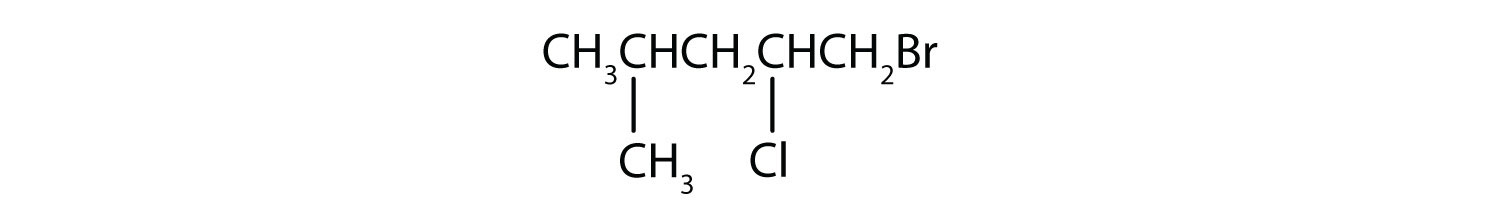
Solutions
5. 2-choro-3-methylbutane
6. 1-bromo-2-chloro-4-methylpentane

