14.2: Reactions with Phosphorus Halides and Thionyl Chloride
- Page ID
- 45248
Carbocation containment
Synthetic organic chemists use phosphorus tribromide and thionyl chloride to convert an alcohol into a better leaving group without carbocation rearrangement.
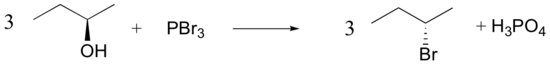
Despite their general usefulness, phosphorous tribromide and thionyl chloride have shortcomings. Hindered 1º- and 2º-alcohols react sluggishly with the former, and may form rearrangement products, as noted in the following equation.
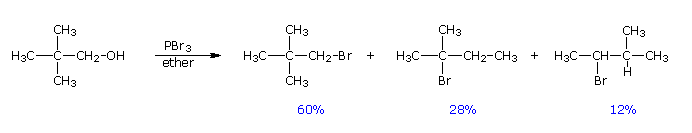
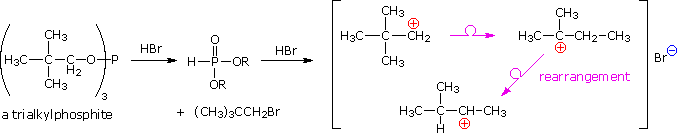
Below, an abbreviated mechanism for the reaction is displayed. The initially formed trialkylphosphite ester may be isolated if the HBr byproduct is scavenged by base. In the presence of HBr a series of acid-base and SN2 reactions take place, along with the transient formation of carbocation intermediates. Rearrangement (pink arrows) of the carbocations leads to isomeric products.
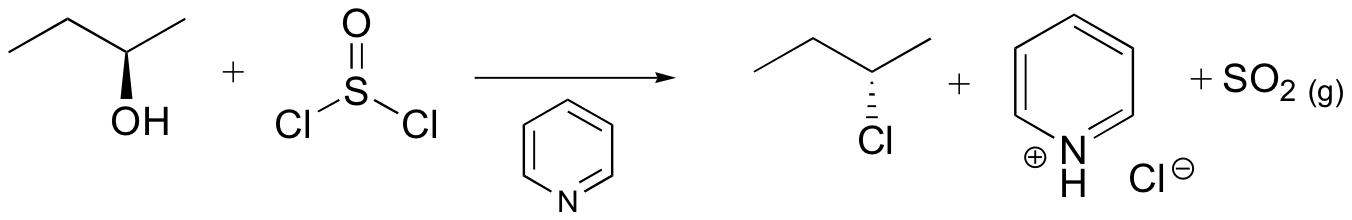
Reaction of thionyl chloride with chiral 2º-alcohols has been observed to proceed with either inversion or retention. In the presence of a base such as pyridine, the intermediate chlorosulfite ester reacts to form an "pyridinium" salt, which undergoes a relatively clean SN2 reaction to the inverted chloride. In ether and similar solvents the chlorosulfite reacts with retention of configuration, presumably by way of a tight or intimate ion pair. This is classified as an SNi reaction (nucleophilic substitution internal). The carbocation partner in the ion pair may also rearrange. These reactions are illustrated by the following equations. An alternative explanation for the retention of configuration, involving an initial solvent molecule displacement of the chlorosulfite group (as SO2 and chloride anion), followed by chloride ion displacement of the solvent moiety, has been suggested. In this case, two inversions lead to retention.

