23.S: Carbonyl Condensation Reactions (Summary)
- Page ID
- 207030
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
Concepts & Vocabulary
- Design multi-step syntheses in which the reactions introduced in this unit are used in conjunction with any of the reactions described in previous units.
- Solve road-map problems that require a knowledge of carbonyl condensation reactions.
- Define, and use in context, any of the key terms introduced.
23.1 Carbonyl Condensations - The Aldol Reaction
- A carbon-carbon bond forming reaction at the alpha carbon through an enolate nucleophile.
- The Aldol Reaction proceeds by first making an enolate nucelophile on an aldehyde (or ketone).
- The enolate nucleophile then dimerizes by attacking the carbonyl of the same aldehyde (or ketone).
- The final product is a beta-hydroxy aldehyde (or ketone).
23.2 Carbonyl Condensations versus Alpha Substitutions
- Carbonyl condensation reactions are a type of alpha substitution reaction.
- Both involve an enolate nucleophile and end with an alpha substitution product.
- In carbonyl condensations, the electrophile is another carbonyl compound.
- Carbonyl condensations are reversible and use a catalytic amount of base.
- The electrophile is already present in the reaction as the enolate is formed in carbonyl condensations.
- Alpha substitution reactions are more directional by design, since a full equivalent of base is used to generate the enolate.
- The electrophile is introduced after the enolate is generated.
23.3 Dehydration of Aldol Products - Synthesis of Enones
- The aldol reaction can undergo a dehydration (loss of water) to yield an α,β-unsaturated aldehyde or ketone.
- The additional stability provided by the conjugated carbonyl system makes some of the products thermodynamically driven.
- The of small products, such as water in this case, are termed condensations, so this reaction is aldol condesation.
- Heat promotes the condensation reactions.
- Under basic conditions, the β-elimination occurs through an E1cb mechanism.
- Under acidic conditions, the β-elimination occurs through an E1 or E2 mechanism.
23.4 Using Aldol Reactions in Synthesis
- If the target contains a β-hydroxy carbonyl compound or an α,β-unsaturated carbonyl compound, then synthetically think aldol reaction or condensation.
- To reverse the aldol condensation, break the enone at the double bond.
- To reverse the aldol reaction, break the C-C bond between the alpha carbon and the carbon attached to the hydroxy group.
- Aldol condensations between different reactants are called mixed or crossed Claisen reactions.
- Multiple products are possible, so the success of the mixed aldol reactions depends on two things:
- The electrophile (or acceptor) is an aldehyde.
- The aldehyde acceptor has no alpha protons.
- Mixed aldols in which both reactants can serve as donors and acceptors generally give complex mixtures of both dimeric (homo) aldols and crossed aldols.
- The aldol condensation of ketones with aryl aldehydes to form α,β-unsaturated derivatives is called the Claisen-Schmidt reaction.
23.6 Intramolecular Aldol Reactions
- Molecules which contain two carbonyl functionalities have the possibility of forming a ring through an intramolecular aldol reaction.
- In these intramolecular reactions, two sets of α-hydrogens need to be considered and most ring forming reaction five and six membered rings are preferred.
23.7 The Claisen Condensation Reaction
- Esters can contain α hydrogens, so can undergo a condensation reaction similar to the aldol reaction called a Claisen Condensation.
- One ester reacts as a nucleophile while the other reacts as an electrophile.
- A new C-C bond is formed in the reaction to form a β-keto ester product.
- An alkoxide that matches the ester group is used to help prevent side reactions in the Claisen Condensation.
- There is a thermodynamic driving step forming an enolate, which is followed by a protonation to obtain the neutral product.
23.8 Mixed Claisen Condensation Reactions
- Claisen condensations between different ester reactants are called Crossed Claisen reactions.
- Crossed Claisen reactions in which both reactants can serve as donors and acceptors generally give complex mixtures.
- Because of this most Crossed Claisen reactions are usually not performed unless one reactant has no alpha hydrogens.
23.9 Intramolecular Claisen Condensation Reactions - The Dieckmann Cyclization
- A diester can undergo an intramolecular reaction called a Dieckmann condensation.
23.10 Conjugate Carbonyl Additions - The Michael Reaction
- In 1,4 additions the nucleophile is added to the carbon β to the carbonyl while the hydrogen is added to the carbon α to the carbonyl.
- Enolates undergo 1,4 addition to α, β-unsaturated carbonyl compounds (product of the Aldol condensation) is a process called a Michael addition.
- A new C-C bond is formed between an enolate and the 4-C of the α, β-unsaturated carbonyl compound.
- The product is a 1,5-dicarbonyl species.
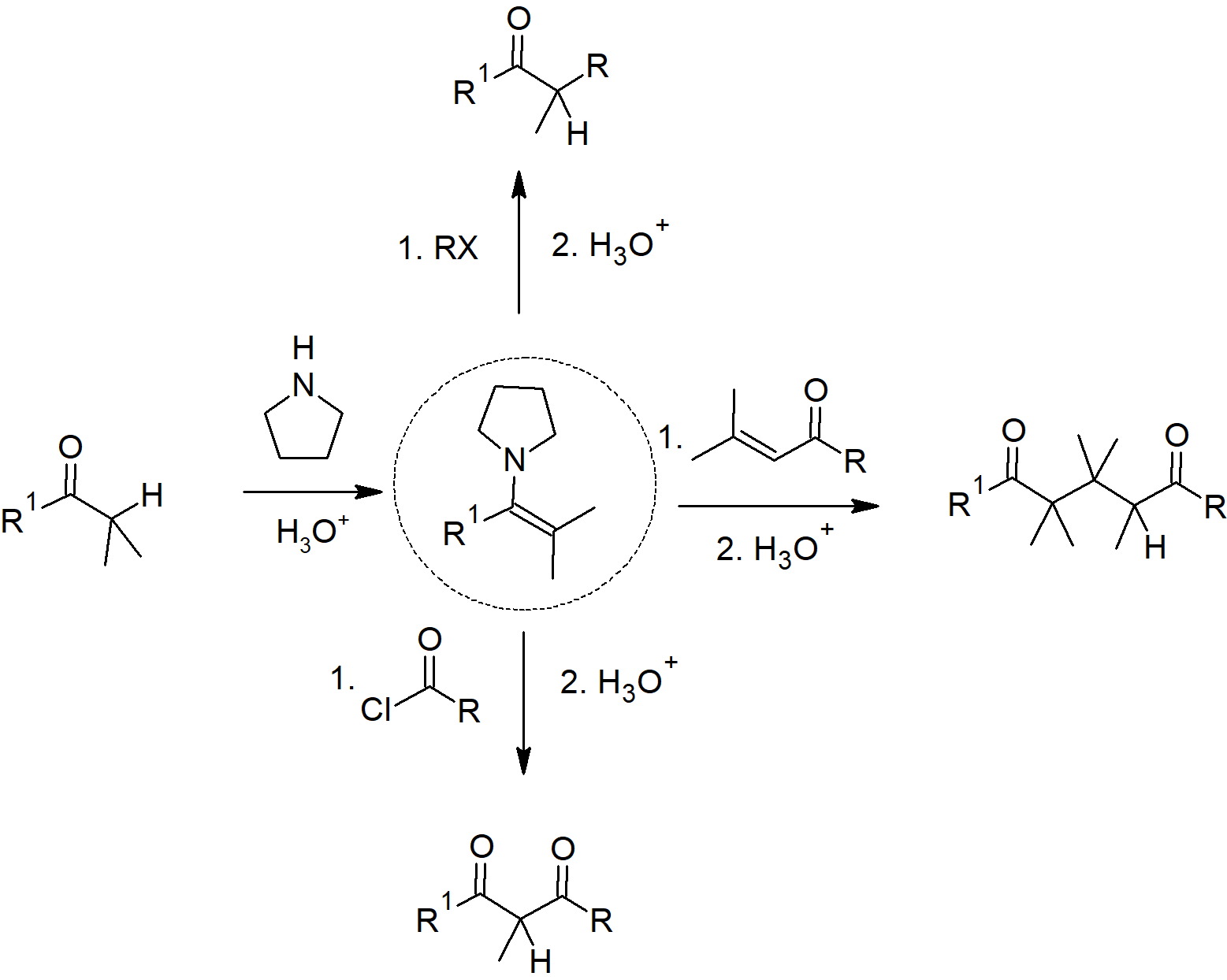
23.11 Carbonyl Condensations with Enamines - The Stork Reaction
- Aldehydes and ketones react with 2o amines to reversibly form enamines.
- Enamines act as nucleophiles similar to enolates.
- This process requires a three steps:
- Formation of the enamine
- Reaction with an eletrophile to form an iminium salt
- Hydrolysis of the iminium salt to reform the aldehyde or ketone
- Advantages of using an enamine over an enolate
- Neutral
- Easier to prepare
- Prevent overreaction issue that occurs when using enolates.
- Enamines undergo an SN2 reaction with alkyl halides to yield the iminium salt.
- Enamines can react with acid halides to form β-dicarbonyls.
- Enamines can also be used as a nucleophile in a Michael reaction.
23.12 The Robinson Annulation Reaction
- Forms a cyclic product from acyclic starting materials by first creating a new C-C bond followed by ring formation.
- The Robinson Annulation reaction starts with a Michael reaction followed by an intramolecular Aldol condensation.
- The formation of 5 or 6 membered rings is preferred.
23.13 Some Biological Carbonyl Condensation Reactions
- Aldol reactions occur in several biological pathways.
- Enzymes that catalyze aldol reactions are called, not surprisingly, 'aldolases'.
- The first step in an aldolase reaction is the deprotonation of an alpha-carbon to generate a nucleophilic carbanion.
- Nature has evolved several distinct strategies to stabilize the intermediate that results.
- Some aldolases use a metal ion to stabilize the negative charge on an enolate intermediate.
- Others catalyze reactions that proceed through neutral Schiff base or enol intermediates.
Skills to Master
- Skill 23.1 Identify the product of an aldol reaction.
- Skill 23.2 Write the detailed mechanism for the aldol reaction.
- Skill 23.3 Describe the difference between a carbonyl condensation reaction and an alpha-substitution reaction.
- Skill 23.4 Know what an enone is.
- Skill 23.5 Write a detailed mechanism for the aldol condensation under basic conditions.
- Skill 23.6 Write a detailed mechanism for the aldol condensation under acidic conditions.
- Skill 23.7 Determine the products of an aldol condensation reaction.
- Skill 23.8 Provide the reactants for a target aldol condensation product.
- Skill 23.9 Write an equation to illustrate a mixed aldol reaction.
- Skill 23.10 Identify the structural features necessary to ensure that two carbonyl compounds will react together in a mixed aldol reaction to give a single product rather than a mixture of products.
- Skill 23.11 Determine whether a given mixed aldol reaction is likely to produce a single product or a mixture of products.
- Skill 23.12 Identify the carbonyl compounds needed to produce a given enone or β‑hydroxy aldehyde or ketone by a mixed aldol reaction.
- Skill 23.13 Write a detailed mechanism for the intramolecular aldol condensation.
- Skill 23.14 Write an equation to illustrate a Claisen condensation reaction.
- Skill 23.15 Write a detailed mechanism for a Claisen condensation reaction or its reverse.
- Skill 23.16 Identify the ester and other reagents needed to form a given β‑keto ester by a Claisen condensation reaction.
- Skill 23.17 Write an equation to illustrate a mixed Claisen condensation.
- Skill 23.18 Identify the structural features that should be present in the two esters if a mixed Claisen condensation is to be successful.
- Skill 23.19 Identify the product formed when a given pair of esters is used in a mixed Claisen condensation.
- Skill 23.20 Identify the esters that should be used to produce a given β‑keto ester by a mixed Claisen condensation.
- Skill 23.21 Write detailed mechanisms for mixed Claisen condensation.
- Skill 23.22 Write a detailed mechanism for an intramolecular Claisen condensation.
- Skill 23.23 Write a detailed mechanism for a given typical Michael reaction.
- Skill 23.24 Identify the product formed in a given Michael reaction.
- Skill 23.25 Identify the reagents necessary to synthesize a given compound by a Michael reaction.
- Skill 23.26 Write a detailed mechanism for each of the three steps of the Stork enamine reaction.
- Skill 23.27 Identify the reagents needed to synthesize a given compound by a Stork enamine reaction.
- Skill 23.28 Write a detailed mechanism for the Robinson Annulation reaction.
- Skill 23.29 Identify the product from an enone and an dicarbonyl under basic conditions.
- Skill 23.30 Identify the steps in which a carbonyl condensation reaction has occurred, given a general outline of a specific biosynthesis.
Summary of Reactions


Stork Enamine Reactions