4.9: Conformations of Polycyclic Molecules
- Page ID
- 31416
After completing this section, you should be able to draw the structures and construct molecular models of simple polycyclic molecules.
Make certain that you can define, and use in context, the key terms below.
- bridgehead carbon atom
- polycyclic molecule
A bridgehead carbon atom is a carbon atom which is shared by at least two rings. The hydrogen atom which is attached to a bridgehead carbon may be referred to as a bridgehead hydrogen.
Note that bicyclo[2.2.1]heptane is the systematic name of norborane. You need not be concerned over the IUPAC name of norbornane. The nomenclature of compounds of this type is beyond the scope of this course.
Nomenclature of Bicyclic Ring Systems
There are many hydrocarbons and hydrocarbon derivatives with two rings having common carbon atoms. There are three main ways that the two rings can be connected. The first is called a fused bicyclic ring structure where the two rings share a covalent bond and a have two bridgehead carbons (marked in red on the structures below). A bridgehead is defined as a carbon that is part of two or more rings. Hydrogens attached to bridge head carbons are often referred to as bridge head hydrogens. The two rings can also be connected by a bridge containing one or more carbons to form a bridged bicyclic molecule. Lastly, the two rings can be joined with a singe bridge head carbon to form spiro bicyclic molecules.
Bicyclic Isomers of C10H18
Naming Fused and Bridged Compounds
Fused and bridged bicyclic compounds are follow similar naming conventions:
- Count the total number of carbons in both rings. This is the parent name. (eg. ten carbons in the system would be decane)
- Count the number of carbons between the bridgeheads, then place the numbers in square brackets in descending order separated by periods. Fused and bridged bycyclic compounds should have three numbers such as [2.2.0]. For fused compounds one of the numbers should be zero.
- Place the word bicyclo at the beginning of the name.
Examples with carbons and hydrogens explicitly shown:
Naming Spiro Compounds
Spiro bicyclics are named using the same basic rules. Because there is only one bridgehead carbon only two numbers will be required in the brackets. Also, the word spiro is placed at the beginning.
Examples
Conformations in Bicyclic Ring Systems
As expected, the connection of two rings has defined effects on the possible conformations. However, the ideas previously discussed in this chapter can be used for conformational analysis. Fused rings have the possibility of two isomers where the bridgehead hydrogens are either cis or trans along the shared bond. These two isomers have significant differences in flexibility and stability as seen in bicyclo[4,4,0]decane more commonly known as decalin. If the positioning of the bridgehead hydrogens are shown in a fused ring the prefix cis or trans should be included in the name.
The trans-isomer is the easiest to describe because the fusion of the two rings creates a rigid, roughly planar, structure made up of two chair conformations. Unlike cyclohexane, the two rings cannot flip from one chair form to another. Accordingly, the orientation of the any substituents is fixed in either an axial or equatorial position in trans-decalin. This means that the C-C bonds coming away from the fused edge are held in equatorial positions relative to each ring thus preventing the possibility of any 1,3-diaxial interactions occurring between ring atoms.
The 3D Structure of Trans-Decalin
The two rings in cis-decalin are also both held in a chair conformations. In comparison, the chair-chair forms of cis-decalin are relatively flexible, and inversion of both rings at once occurs fairly easily.
The 3D Structure of Cis-Decalin
The flexibility of cis-decalin allows for a substituent to interconvert between axial and equatorial conformations. In much the same fashion as cyclohexane, equatorial substituents tend to create less steric strain and create a more stable conformer.
A major difference in cis-decalin is the fact that one of C-C bonds coming away from the fused edge is held an an axial position. This is true in both ring-flip conformations. This axial C-C bond causes 1,3-diaxial interactions to occur in cis-decalin making it roughly 8.4 kJ/mol less stable than trans-decalin. This amount of 1,3-diaxial steric strain is roughly equivalent to that of an ethyl substituent attached to a cyclohexane ring (8.0 kJ/mol)
Bicyclic compounds with a bridge typically have very little flexibility and are often held in a ridged conformation. The molecule norbornane represent a cyclohexane ring connected by a single carbon bridge.
The 3D Structure of Norbornane
Norbornane is estimated to have 72 kJ/mol of ring strain which can be understood when viewing the contained rings. The carbon bridge in norbornane holds the cyclohexane ring at the bottom in a boat conformation creating torsional strain from eclipsing bonds along the edge.
Also, the carbon bridge forms a cyclopentane ring (shown in red below making up the right side of the structure) with increased angle strain throughout the whole molecule.
Polycyclic Systems in Nature
Fused ring systems like decalin are very common in natural products. In fact, similar ring systems are found in steroids, which are an important class of lipids. Steroids generally have structures that include three six-membered rings and a five-membered ring connected by three fused bonds. Most natural steroids have a trans configuration at all three fusion points. This tends to give steroids a rigid and semi-flat structure.
Sex hormones are an example of steroids. The primary male hormone, testosterone, is responsible for the development of secondary sex characteristics. Two female sex hormones, progesterone and estrogen (or estradiol) control the ovulation cycle. Notice that the male and female hormones have only slight differences in structures, but yet have very different physiological effects. Testosterone promotes the normal development of male genital organs and is synthesized from cholesterol in the testes. It also promotes secondary male sexual characteristics such as deep voice, facial and body hair.
The 3D Structure of Estradiol
The best known and most abundant steroid in the body is cholesterol. Cholesterol is formed in brain tissue, nerve tissue, and the blood stream. It is the major compound found in gallstones and bile salts. Cholesterol also contributes to the formation of deposits on the inner walls of blood vessels. These deposits harden and obstruct the flow of blood. This condition, known as atherosclerosis, results in various heart diseases, strokes, and high blood pressure.
Exercises
1)
i)
j)
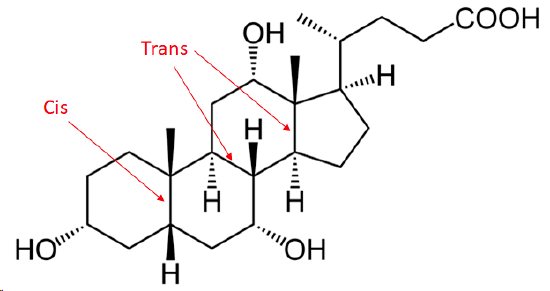
3) The following molecule is cholic acid. Determine if the three fused bonds have a cis or trans configuration.
Solutions
1)
a) Bicyclo[2.1.1]hexane
b) Bicyclo[3.2.1]octane
c) Bicyclo[2.1.0]pentane (more commonly called "housane")
d) Bicyclo[2.2.2]octane
e) cis-Bicyclo[3.3.0]octane
f) cis-Bicyclo[1.1.0]butane
g) Bicyclo[1.1.1]pentane
h) Bicyclo[4.3.3]dodecane
i) Spiro[5.2]octane
j) Spiro[3.3]heptane
2)