19.5: Halogenation of the α- Carbon and Aldehydes and Ketones
- Page ID
- 13988
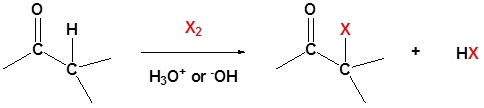
A carbonyl containing compound with \(\alpha\) hydrogens can undergo a substitution reaction with halogens. This reaction comes about because of the tendency of carbonyl compounds to form enolates in basic condition and enols in acidic condition. In these cases even weak bases, such as the hydroxide anion, is sufficient enough to cause the reaction to occur because it is not necessary for a complete conversion to the enolate. For this reaction Cl2, Br2 or I2 can be used as the halogens.
General reaction
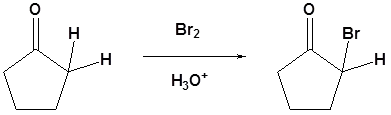
Acid Catalyzed Mechanism
Under acidic conditions the reaction occurs thought the formation of an enol which then reacts with the halogen.
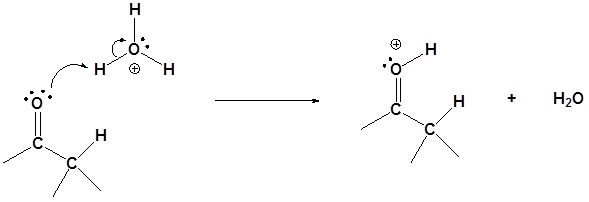
Step 1: Protonation of the carbonyl
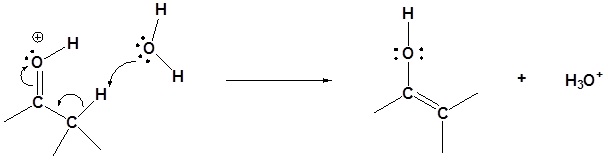
Step 2: Enol formation
Step 3: SN2 attack
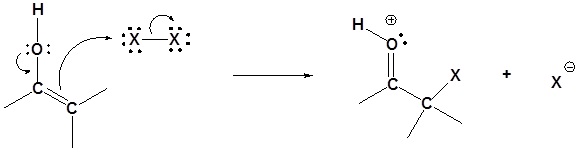
Step 4: Deprotonation
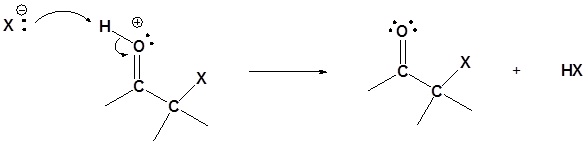
Base Catalyzed Mechanism
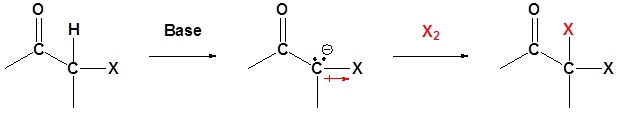
Under basic conditions the enolate forms and then reacts with the halogen. Note! This is base promoted and not base catalyzed because an entire equivalent of base is required.
Step 1: Enolate formation
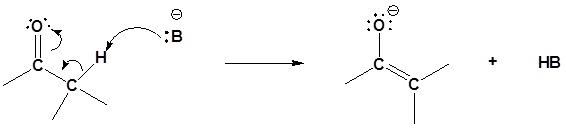
Step 2: SN2 attack
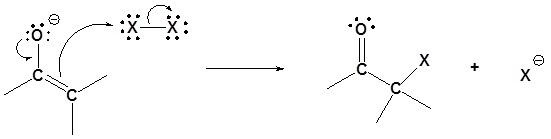
Overreaction during base promoted α halogenation
The fact that an electronegative halogen is placed on an α carbon means that the product of a base promoted α halogenation is actually more reactive than the starting material. The electron withdrawing effect of the halogen makes the α carbon even more acidic and therefor promotes further reaction. Because of this multiple halogenations can occur. This effect is exploited in the haloform reaction discussed later. If a monohalo product is required then acidic conditions are usually used.
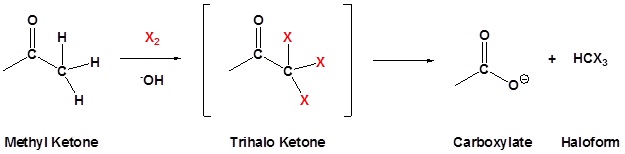
The Haloform Reaction
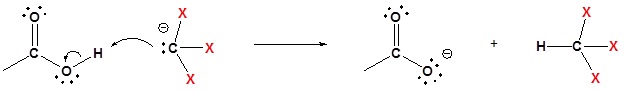
Methyl ketones typically undergo halogenation three times to give a trihalo ketone due to the increased reactivity of the halogenated product as discussed above. This trihalomethyl group is an effective leaving group due to the three electron withdrawing halogens and can be cleaved by a hydroxide anion to effect the haloform reaction. The product of this reaction is a carboxylate and a haloform molecule (CHCl3, CHBr3, CHI3). Overall the haloform reaction represents an effective method for the conversion of methyl ketones to carboxylic acids. Typically, this reaction is performed using iodine because the subsequent iodoform (CHI3) is a bright yellow precipitate which is easily filtered off.
General reaction
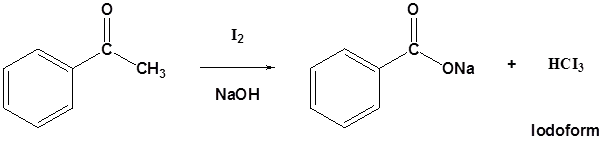
Mechanism
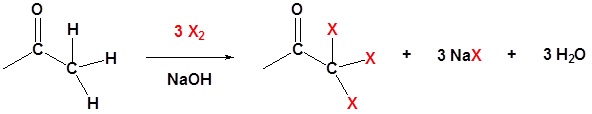
1) Formation of the trihalo species
2) Nulceophilic attack on the carbonyl carbon
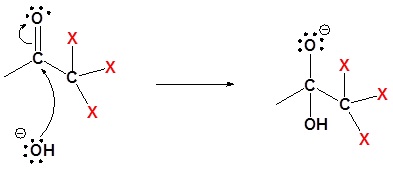
3) Removal of the leaving group
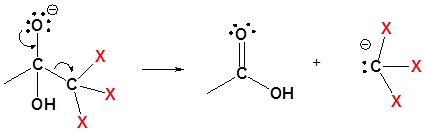
4) Deprotonation
Please draw the products of the following reactions
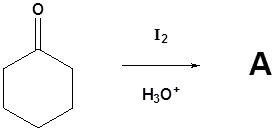
- Answer
-


