21.5: Reactions of Carboxylic Acids Overview
- Page ID
- 45923
Carboxylic Acid Reactions Overview
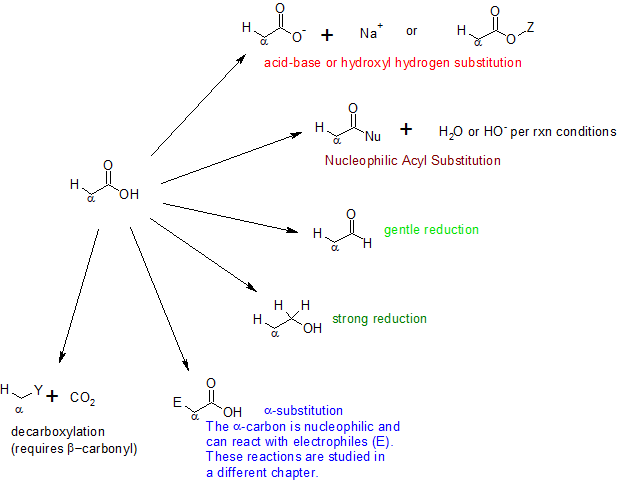
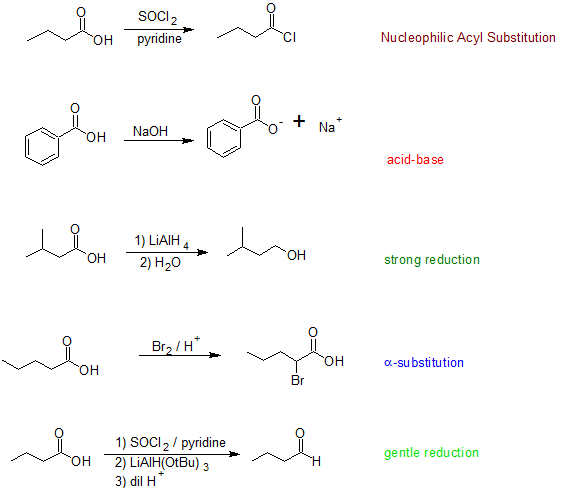
Four general reaction categories are represented here: (1) As carboxylic acid deprotonates quite readily, it is quite easy to form a carboxylate salt or to substitute the hydroxyl hydrogen. (2) The category of nucleophilic acyl substitution represents the substitution of the whole hydroxyl group, which we will see later in more detail leads to several carboxylic acid derivatives (e.g. acid halides, esters, amides, thioesters, acid anhydrides etc.). (3) Like other carbonyl compounds, carboxylic acids can be reduced by reagents like LiAlH4. (4) While the proton on the carbon alpha to the carbonyl group is not as acidic as the hydroxyl hydrogen, it can be removed leading to substitution at the alpha site.
The scheme summarizes some of the general reactions that carboxylic acids undergo. The reaction details can be found in other sections of this text.
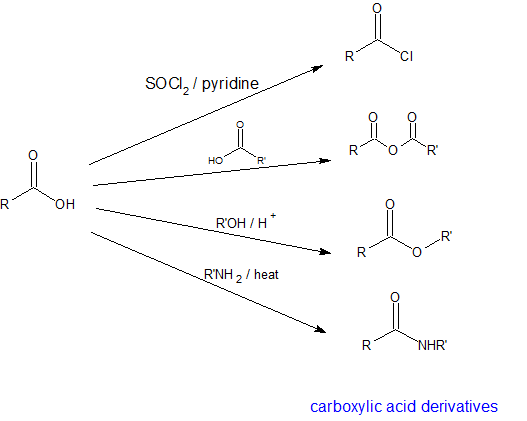
Nucleophilic Acyl Substitution Reactions and the Carboxylic Acid Derivatives
In subsequent sections, the reaction details to synthesize acyl chlorides, anhydrides, esters, and amides from carboxylic acids will be discussed. Because acyl chlorides, anhydrides, esters, and amides can all be synthesized from carboxylic acids, these functional groups are called "carboxylic acid derivatives".
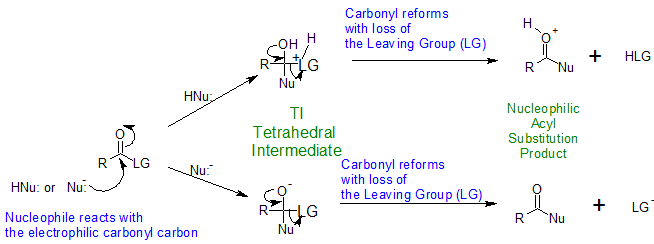
These reactions all share the same general mechanism. The nucleophile reacts with the electrophilic carbonyl carbon to produce the "Tetrahedral Intermediate or TI". The carbonyl reforms forcing the loss of the leaving group (LG) to produce the "Nucleophilic Acyl Substitution Product". The reaction mechanism depends on the pH of the reaction conditions. The reaction mechanisms for both acidic and basic conditions are shown below.
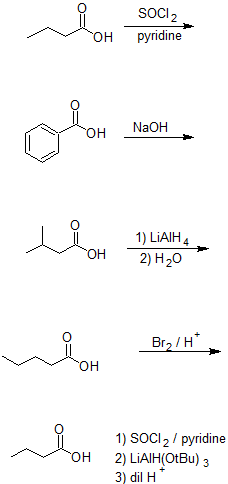
Exercise
5. Draw the bond-line structures for the products and classify each reaction using the terms from the top diagram:
acid-base, NAS (Nucleophilic Acyl Substitutioin), gentle reduction, strong reduction, or alpha-substitution.
- Answer
-
5.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

