19.3: Review of Ketone and Aldehyde Synthesis
- Page ID
- 45592
So far this text has discussed aldehyde and ketone synthesis from the ozolysis of alkenes, hydration of alkynes, oxidation of alcohols, and Friedel-Crafts acylation of benzene rings.
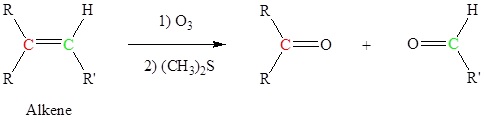
Alkenes can be cleaved using ozone (O3) to form aldehydes and/or ketones
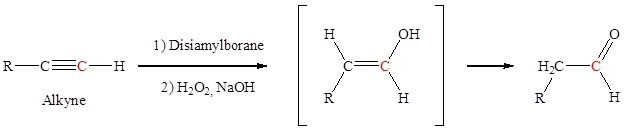
Hydration of an alkyne to form aldehydes
Anti-Markovnikov addition of a hydroxyl group to an alkyne forms an aldehyde. The addition of a hydroxyl group to an alkyne causes tautomerization which subsequently forms a carbonyl.
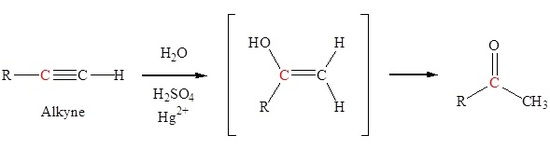
Hydration of an alkyne to form ketones
The addition of a hydroxyl group to an alkyne causes tautomerization which subsequently forms a carbonyl. Markovnikov addition of a hydroxyl group to an alkyne forms a ketone.
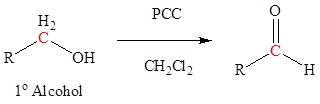
Oxidation of 1o alcohols with PCC to form aldehydes
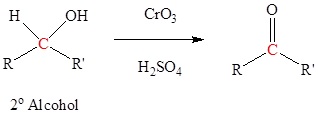
Oxidation of 2o alcohols to form ketones
Typically uses Jones reagent (CrO3 in H2SO4) but many other reagents can be used
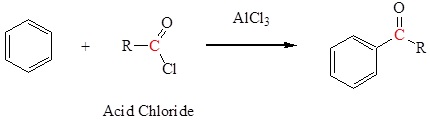
Friedel-Crafts acylation to form a ketone
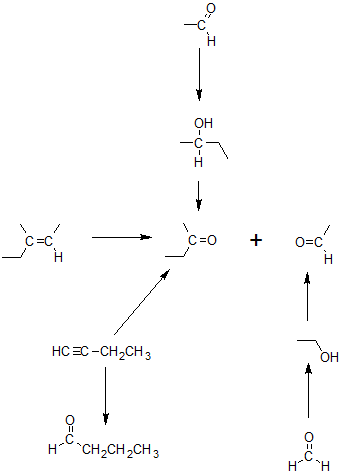
Exercise
2. Specify the reagents to complete the reaction map below.
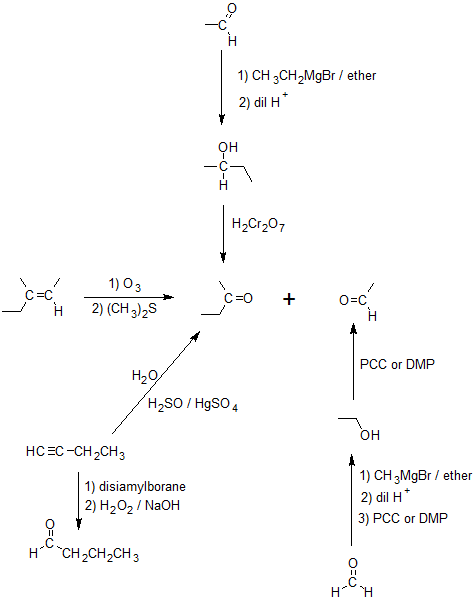
- Answer
-
2.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)

