14.4: Dehydration Reactions of Alcohols
- Page ID
- 45250
Dehydration of Alcohols to Yield Alkenes
One way to synthesize alkenes is by dehydration of alcohols, a process in which alcohols undergo E1 or E2 mechanisms to lose water and form a double bond. The dehydration reaction of alcohols to generate alkene proceeds by heating the alcohols in the presence of a strong acid, such as sulfuric or phosphoric acid, at high temperatures.
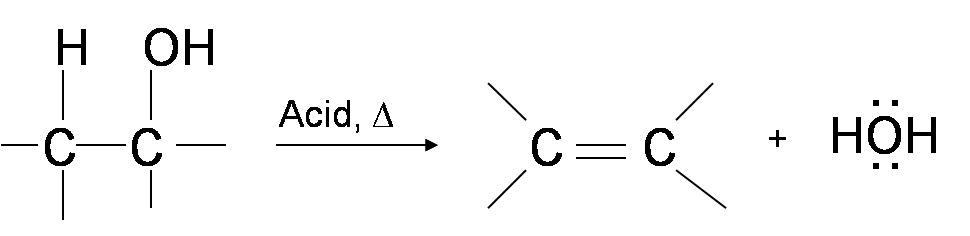
The required range of reaction temperature decreases with increasing substitution of the hydroxy-containing carbon:
- 1° alcohols: 170° - 180°C
- 2° alcohols: 100°– 140 °C
- 3° alcohols: 25°– 80°C
If the reaction is not sufficiently heated, the alcohols do not dehydrate to form alkenes, but react with one another to form ethers (e.g., the Williamson Ether Synthesis).
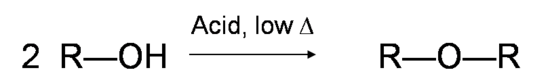
Alcohols are amphoteric; they can act as both acid or base. The lone pair of electrons on oxygen atom makes the –OH group weakly basic. Oxygen can donate two electrons to an electron-deficient proton. Thus, in the presence of a strong acid, R—OH acts as a base and protonates into the very acidic alkyloxonium ion +OH2 (The pKa value of a tertiary protonated alcohol can go as low as -3.8). This basic characteristic of alcohol is essential for its dehydration reaction with an acid to form alkenes.
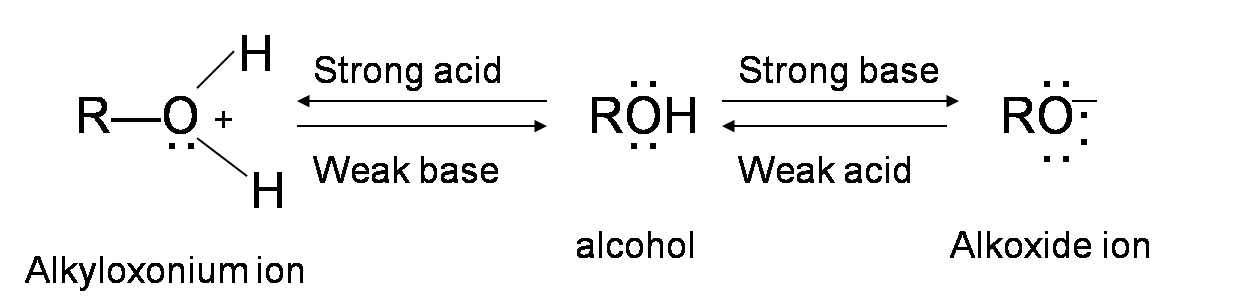
Mechanism for the Dehydration of Alcohol into Alkene
Different types of alcohols may dehydrate through a slightly different mechanism pathway. However, the general idea behind each dehydration reaction is that the –OH group in the alcohol donates two electrons to H+ from the acid reagent, forming an alkyloxonium ion. This ion acts as a very good leaving group which leaves to form a carbocation. The deprotonated acid (the base) then reacts with the hydrogen adjacent to the carbocation and form a double bond.
Primary alcohols undergo bimolecular elimination (E2 mechanism) while secondary and tertiary alcohols undergo unimolecular elimination (E1 mechanism). The relative reactivity of alcohols in dehydration reactions is ranked as follows:
Methanol < primary < secondary < tertiary
Primary alcohols dehydrate through the E2 mechanism. The hydroxyl oxygen donates two electrons to a proton from sulfuric acid (H2SO4), forming an alkyloxonium ion. Then the conjugate base, HSO4–, reacts with one of the adjacent (beta) hydrogen atoms while the alkyloxonium ion leaves in a concerted process, forming a double bond.

Secondary and tertiary alcohols dehydrate through the E1 mechanism. Similarly to the reaction above, secondary and tertiary –OH protonate to form alkyloxonium ions. However, in this case the ion leaves first and forms a carbocation as the reaction intermediate. The water molecule (which is a stronger base than the HSO4- ion) then abstracts a proton from an adjacent carbon to form a double bond. Notice in the mechanism below that the alkene formed depends on which proton is abstracted: the red arrows show formation of the more substituted 2-butene, while the blue arrows show formation of the less substituted 1-butene. Recall that according to Zaitsev's Rule, the more substituted alkenes are formed preferentially because they are more stable than less substituted alkenes. Additinally, trans alkenes are more stable than cis alkenes and are also the major product formed. For the example below, the trans diastereomer of the 2-butene product is most abundant.
Dehydration reaction of secondary alcohol
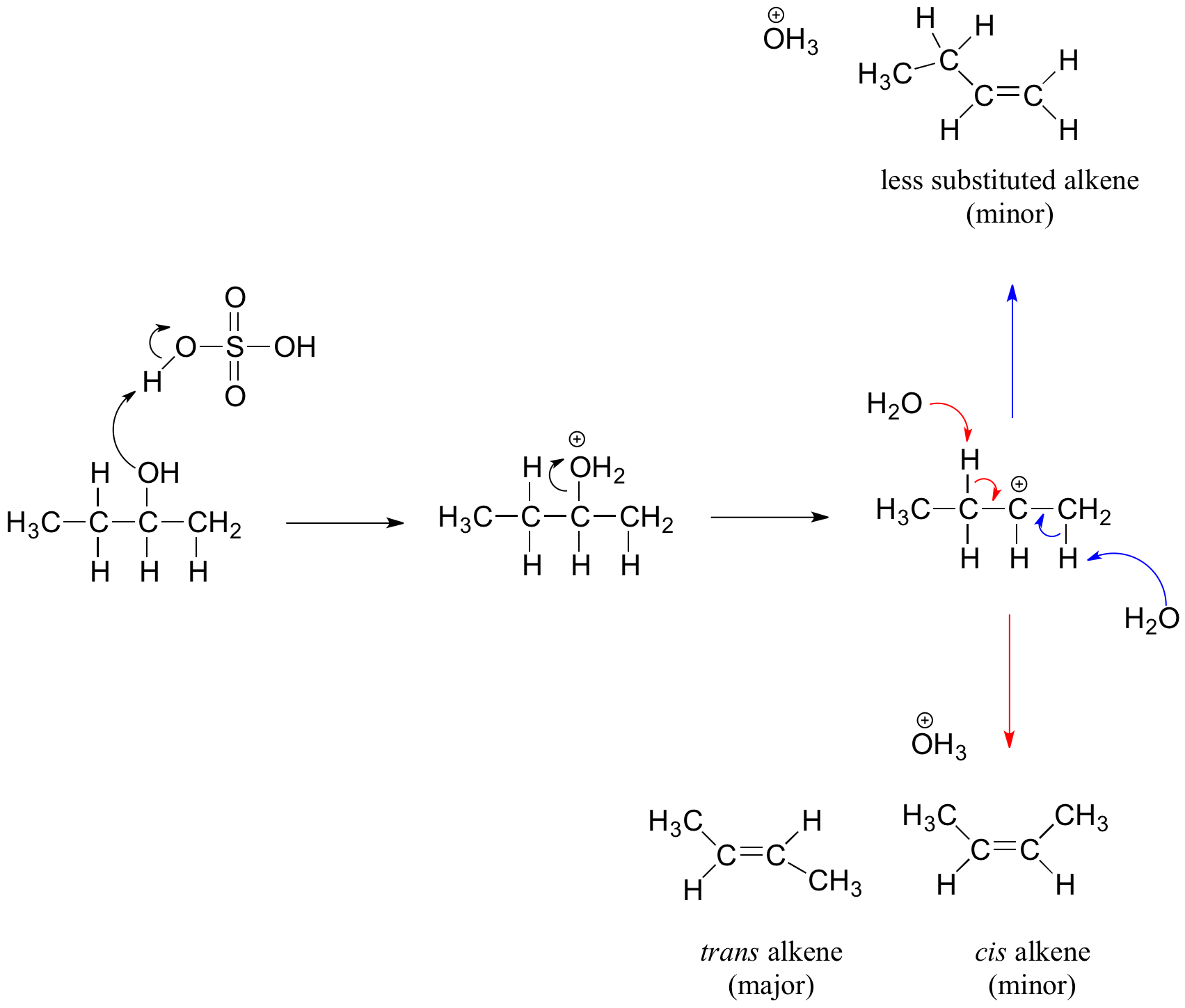
The dehydration mechanism for a tertiary alcohol is analogous to that shown above for a secondary alcohol.
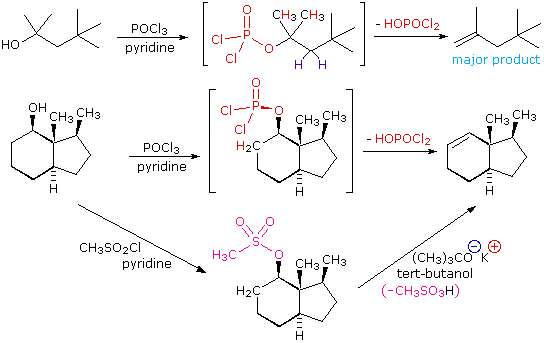
The E2 elimination of 3º-alcohols under relatively non-acidic conditions may be accomplished by treatment with phosphorous oxychloride (POCl3) in pyridine. This procedure is also effective with hindered 2º-alcohols, but for unhindered and 1º-alcohols an SN2 chloride ion substitution of the chlorophosphate intermediate competes with elimination. Examples of these and related reactions are given in the following figure. The first equation shows the dehydration of a 3º-alcohol. The predominance of the non-Zaitsev product (less substituted double bond) is presumed due to steric hindrance of the methylene group hydrogen atoms, which interferes with the approach of base at that site. The second example shows two elimination procedures applied to the same 2º-alcohol. The first uses the single step POCl3 method, which works well in this case because SN2 substitution is retarded by steric hindrance. The second method is another example in which an intermediate sulfonate ester confers halogen-like reactivity on an alcohol. In every case the anionic leaving group is the conjugate base of a strong acid.
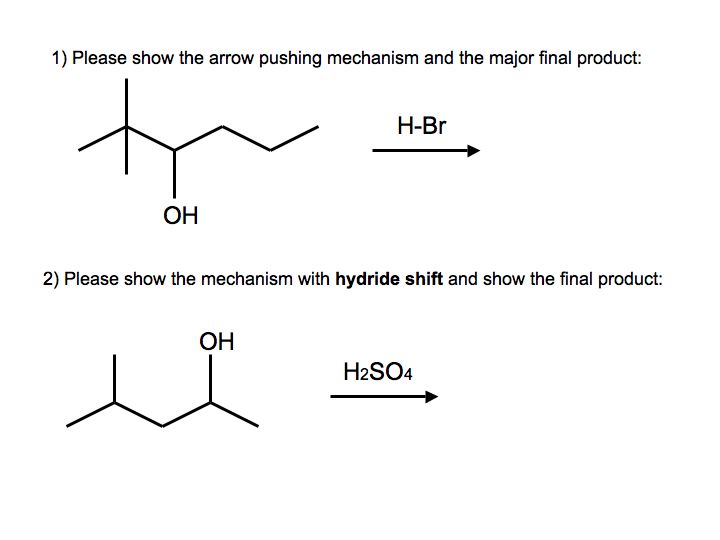
Practice Problems (aka Exercises)

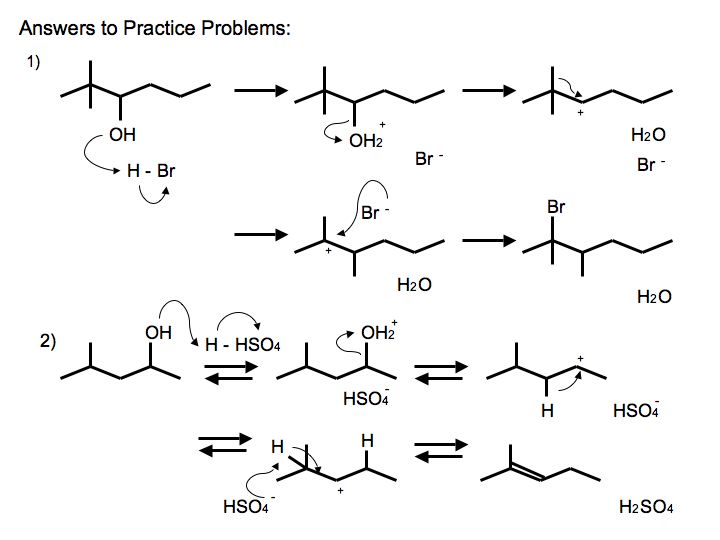
.jpg?revision=2)
Exercises
6. Starting with cyclohexanol, describe how you would prepare cyclohexene.
7. In the dehydration of 1-methylcyclohexanol, which product is favored?
8.
In the dehydration of this diol the resulting product is a ketone. Draw the mechanism of its formation. (Hint a rearrangement occurs)
9.
Draw an arrow pushing mechanism for the acid catalyzed dehydration of the following alcohol, make sure to draw both potential mechanisms. Assume no rearrangement for the first two product mechanisms. Which of these two would likely be the major product? If there was a rearrangement, draw the expected major product.
Answer
-
6. H2SO4 with heat since there are no concerns about C+ rearrangement
7. The more substituted alkene is favored, as more substituted alkenes are relatively lower in energy.
8.
This reaction is known as the Pinacol rearrangement.
Note how the carbocation after the rearrangement is resonance stabilized by the oxygen
9. Note: While the mechanism is instructive for the first part of the this answer. The carbocation rearrangement would occur and determine the major and minor products as explained in the second part of this answer.
The major product of this mechanism would be the more highly substituted alkene, or the product formed from the red arrows.
Note: With the secondary carbocation adjacent a tertiary carbon center, a 1,2 hydride shift (rearrangement) would occur to form a tertiary carbocation and vcompound below would be the major product. The minor product being the same product as the one formed from the red arrows.
Contributors and Attributions
- Jeffrey Ma

