14.1: Reactions of Alcohols with Hydrohalic Acids
- Page ID
- 110489
Conversion of Alcohols into Alkyl Halides
When alcohols react with a hydrogen halide, a substitution takes place producing an alkyl halide and water:
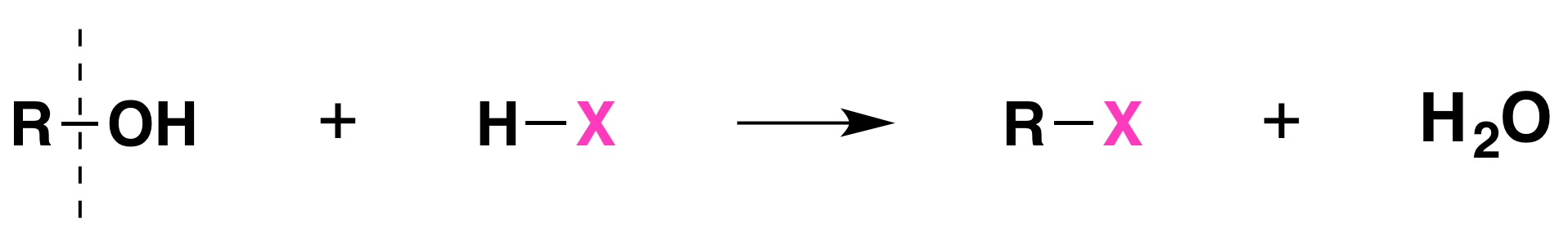
- The order of reactivity of alcohols is 3° > 2° > 1° methyl.
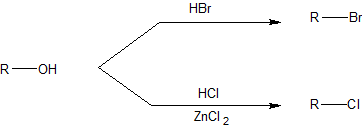
- The order of reactivity of the hydrogen halides is HI > HBr > HCl (HF is generally unreactive).
The reaction is acid catalyzed. Alcohols react with the strongly acidic hydrogen halides HCl, HBr, and HI, but they do not react with nonacidic NaCl, NaBr, or NaI. Primary and secondary alcohols can be converted to alkyl chlorides and bromides by allowing them to react with a mixture of a sodium halide and sulfuric acid:
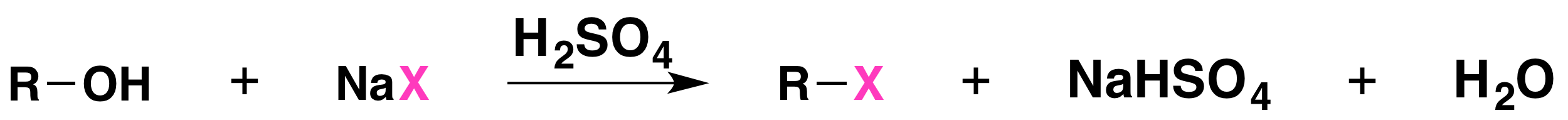
Because Cl- is a weaker nucleophile than Br-, the reaction with HCl requires a catalyst such as ZnCl2 as shown below.
Mechanisms of the Reactions of Alcohols with HX
Secondary, tertiary, allylic, and benzylic alcohols appear to react by a mechanism that involves the formation of a carbocation, in an \(S_N1\) reaction with the protonated alcohol acting as the substrate.
The \(S_N1\) mechanism is illustrated by the reaction tert-butyl alcohol and aqueous hydrochloric acid (\(H_3O^+\), \(Cl^-\) ). The first two steps in this \(S_n1\) substitution mechanism are protonation of the alcohol to form an oxonium ion. Although the oxonium ion is formed by protonation of the alcohol, it can also be viewed as a Lewis acid-base complex between the cation (\(R^+\)) and \(H_2O\). Protonation of the alcohol converts a poor leaving group (OH-) to a good leaving group water, H2O, which makes the dissociation step of the \(S_N1\) mechanism more favorable.
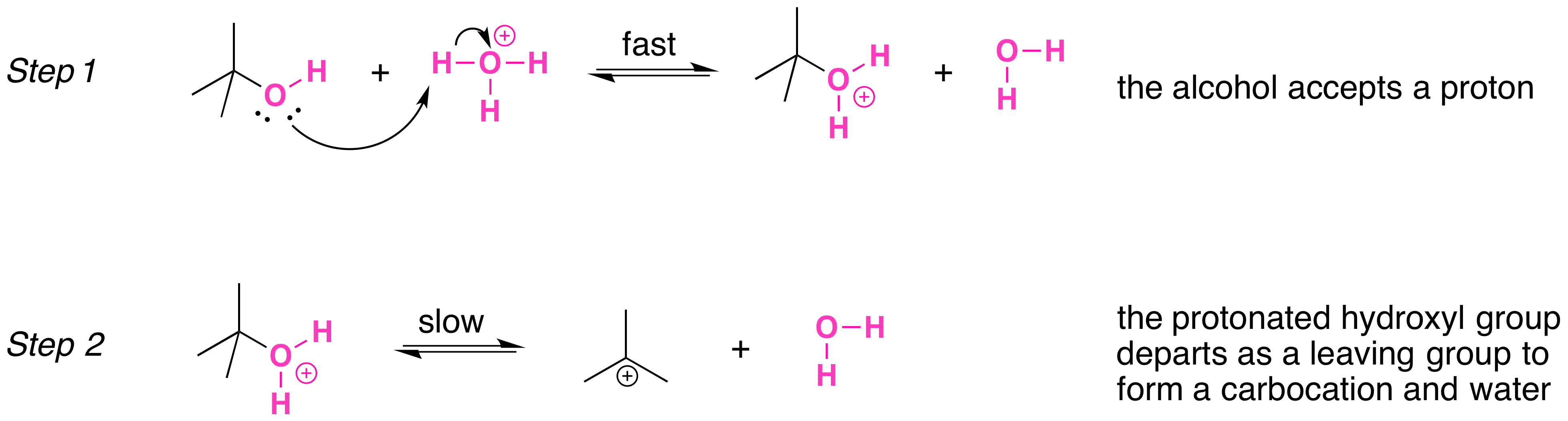
In step 3, the carbocation reacts with a nucleophile (a halide ion) to complete the substitution.

When we convert an alcohol to an alkyl halide, we carry out the reaction in the presence of acid and in the presence of halide ions, and not at elevated temperature. Halide ions are good nucleophiles (they are much stronger nucleophiles than water), and since halide ions are present in high concentration, most of the carbocations react with an electron pair of a halide ion to form a more stable species, the alkyl halide product. The overall result is an SN1 reaction.
Not all acid-catalyzed conversions of alcohols to alkyl halides proceed through the formation of carbocations. Primary alcohols and methanol react to form alkyl halides under acidic conditions by an SN2 mechanism.
In these reactions the function of the acid is to produce a protonated alcohol. The halide ion then displaces a molecule of water (a good leaving group) from carbon; this produces an alkyl halide:
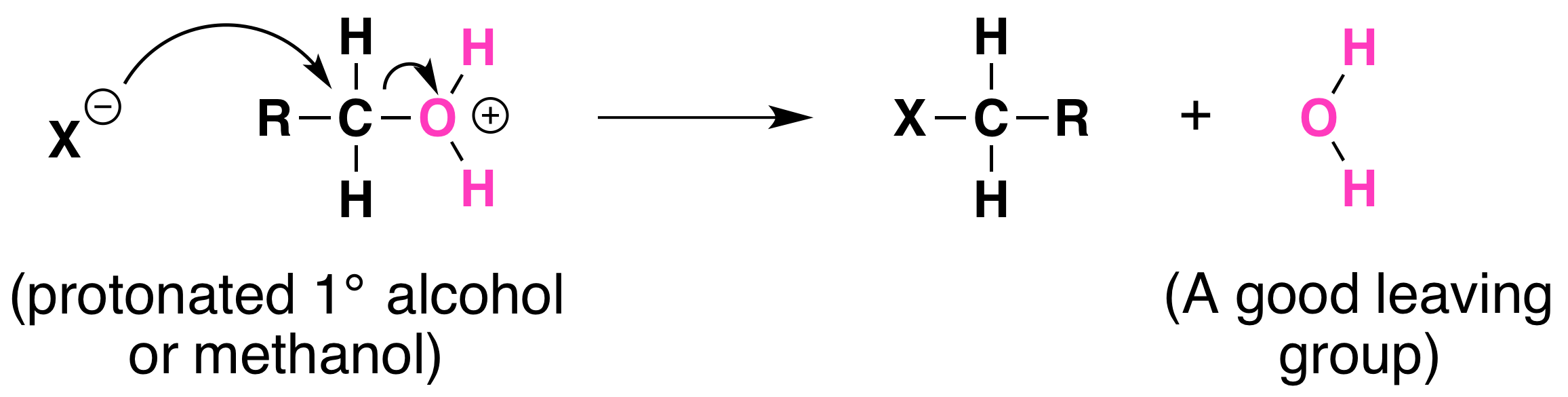
Again, acid is required. Although halide ions (particularly iodide and bromide ions) are strong nucleophiles, they are not strong enough to carry out substitution reactions with alcohols themselves. Direct displacement of the hydroxyl group does not occur because the leaving group would have to be a strongly basic hydroxide ion:
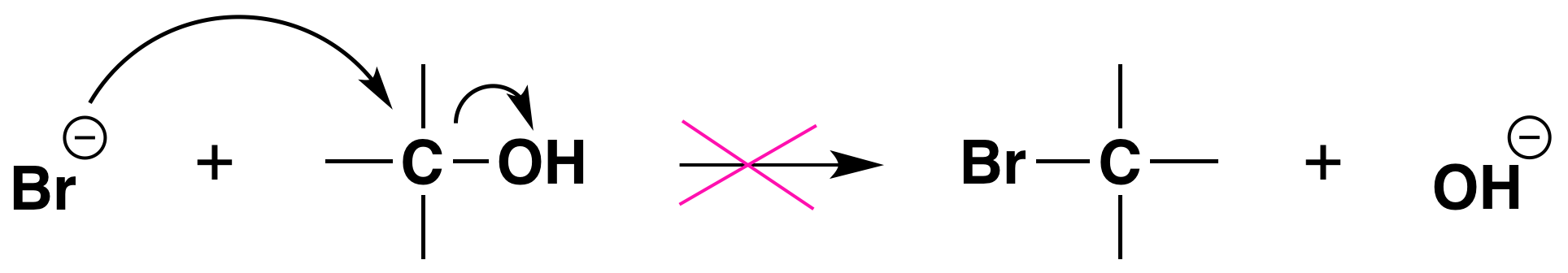
We can see now why the reactions of alcohols with hydrogen halides are acid-promoted.
Carbocation rearrangements are extremely common in organic chemistry reactions are are defined as the movement of a carbocation from an unstable state to a more stable state through the use of various structural reorganizational "shifts" within the molecule. Once the carbocation has shifted over to a different carbon, we can say that there is a structural isomer of the initial molecule. However, this phenomenon is not as simple as it sounds.
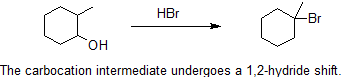
The most common methods for converting 1º- and 2º-alcohols to the corresponding chloro and bromo alkanes (i.e. replacement of the hydroxyl group) are treatments with thionyl chloride and phosphorus tribromide, respectively. These reagents are generally preferred over the use of concentrated HX due to the harsh acidity of these hydrohalic acids and the carbocation rearrangements associated with their use. The alcohol reactions with thionyl chloride or phosphorus tribromide are discussed in the next section.
Exercises
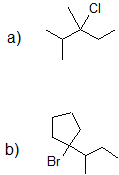
1. Predict the product of each reaction below.
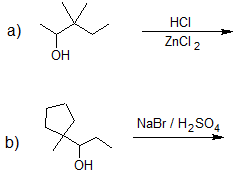
- Answer
-
1.
Contributors and Attributions
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry

