13.3: Physical Properties of Alcohols
- Page ID
- 45232
Boiling Points
The chart below shows the boiling points of the following simple primary alcohols with up to 4 carbon atoms:

These boiling points are compared with those of the equivalent alkanes (methane to butane) with the same number of carbon atoms.
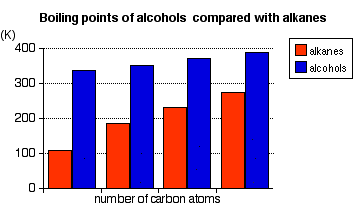
Notice that:
- The boiling points of the alcohols increase as the number of carbon atoms increases.
The patterns in boiling point reflect the patterns in intermolecular attractions.
Hydrogen bonding
Hydrogen bonding occurs between molecules in which a hydrogen atom is attached to a strongly electronegative element: fluorine, oxygen or nitrogen. In the case of alcohols, hydrogen bonds occur between the partially-positive hydrogen atoms and lone pairs on oxygen atoms of other molecules.
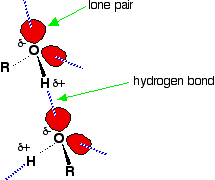
The hydrogen atoms are slightly positive because the bonding electrons are pulled toward the very electronegative oxygen atoms. In alkanes, the only intermolecular forces are van der Waals dispersion forces. Hydrogen bonds are much stronger than these, and therefore it takes more energy to separate alcohol molecules than it does to separate alkane molecules. This the main reason for higher boiling points in alcohols.
The effect of van der Waals forces
- Comparison between alkanes and alcohols: Even without any hydrogen bonding or dipole-dipole interactions, the boiling point of the alcohol would be higher than the corresponding alkane with the same number of carbon atoms.
Compare ethane and ethanol:
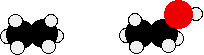
Ethanol is a longer molecule, and the oxygen atom brings with it an extra 8 electrons. Both of these increase the size of the van der Waals dispersion forces, and subsequently the boiling point. A more accurate measurement of the effect of the hydrogen bonding on boiling point would be a comparison of ethanol with propane rather than ethane. The lengths of the two molecules are more similar, and the number of electrons is exactly the same.
Solubility of alcohols in water
Small alcohols are completely soluble in water; mixing the two in any proportion generates a single solution. However, solubility decreases as the length of the hydrocarbon chain in the alcohol increases. At four carbon atoms and beyond, the decrease in solubility is noticeable; a two-layered substance may appear in a test tube when the two are mixed.
Consider ethanol as a typical small alcohol. In both pure water and pure ethanol the main intermolecular attractions are hydrogen bonds.
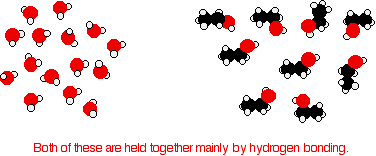
In order to mix the two, the hydrogen bonds between water molecules and the hydrogen bonds between ethanol molecules must be broken. Energy is required for both of these processes. However, when the molecules are mixed, new hydrogen bonds are formed between water molecules and ethanol molecules.
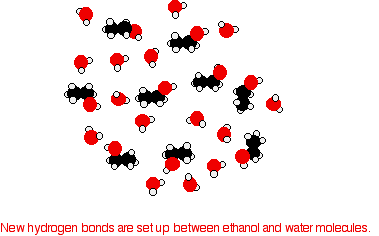
The energy released when these new hydrogen bonds form approximately compensates for the energy needed to break the original interactions. In addition, there is an increase in the disorder of the system, an increase in entropy. This is another factor in deciding whether chemical processes occur. Consider a hypothetical situation involving 5-carbon alcohol molecules.
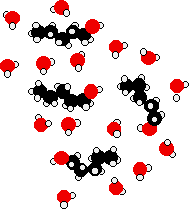
The hydrocarbon chains are forced between water molecules, breaking hydrogen bonds between those water molecules. The -OH ends of the alcohol molecules can form new hydrogen bonds with water molecules, but the hydrocarbon "tail" does not form hydrogen bonds. This means that many of the original hydrogen bonds being broken are never replaced by new ones.
In place of those original hydrogen bonds are merely van der Waals dispersion forces between the water and the hydrocarbon "tails." These attractions are much weaker, and unable to furnish enough energy to compensate for the broken hydrogen bonds. Even allowing for the increase in disorder, the process becomes less feasible. As the length of the alcohol increases, this situation becomes more pronounced, and thus the solubility decreases.
Exercise
Use 1-octanol and ethanol, shown below, to answer the following questions.
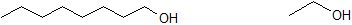
3. Which compound is more water soluble?
4. Which compound has the highest boiling point?
5. Explain why the answers above are not in conflict using your understanding of intermolecular forces, relative boiling points, and solubility.
- Answer
-
3. ethanol
4. octanol
5. While both compounds exhibit H-bonding, the smaller, hydrophobic carbon chain of ethanol results in higher water solubility of ethanol while the longer carbon chain of octanol increases the surface area resulting in a higher boiling point.
Contributors
Dr. Dietmar Kennepohl FCIC (Professor of Chemistry, Athabasca University)
Prof. Steven Farmer (Sonoma State University)
William Reusch, Professor Emeritus (Michigan State U.), Virtual Textbook of Organic Chemistry
Jim Clark (Chemguide.co.uk)
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."

