20.17: Reactions of Acid Chlorides
- Page ID
- 30900
Acyl chlorides (also known as acid chlorides) are one of a number of types of compounds known as "acid derivatives". This is ethanoic acid:
If you remove the -OH group and replace it by a -Cl, you have produced an acyl chloride.
This molecule is known as ethanoyl chloride and for the rest of this topic will be taken as typical of acyl chlorides in general. Acyl chlorides are extremely reactive. They are open to attack by nucleophiles - with the overall result being a replacement of the chlorine by something else.
Why are acyl chlorides attacked by nucleophiles?
The carbon atom in the -COCl group has both an oxygen atom and a chlorine atom attached to it. Both of these are very electronegative. They both pull electrons towards themselves, leaving the carbon atom quite positively charged.
The Overall Reaction
We are going to generalize this for the moment by writing the reacting molecule as "Nu-H". Nu is the bit of the molecule which contains the nucleophilic oxygen or nitrogen atom. The attached hydrogen turns out to be essential to the reaction. The general equation for the reaction is:
In each case, the net effect is that you replace the -Cl by -Nu, and hydrogen chloride is formed as well.
Since the initial attack is by a nucleophile, and the overall result is substitution, it would seem reasonable to describe the reaction as nucleophilic substitution. However, the reaction happens in two distinct stages. The first involves an addition reaction, which is followed by an elimination reaction where HCl is produced. So the mechanism is also known as nucleophilic addition / eliminatio
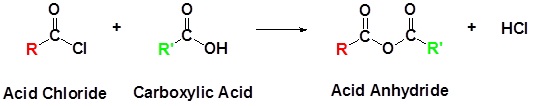
Acid chlorides react with carboxylic acids to form anhydrides.
General Reaction
| Example 1: |
|---|
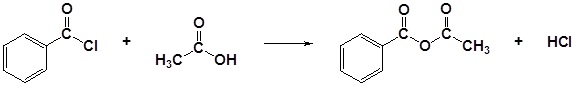 |
Mechanism
1) Nucleophilic attack by the alcohol
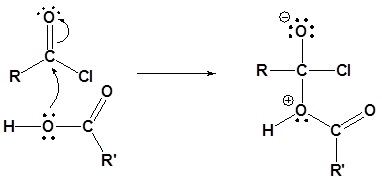
2) Leaving group is removed
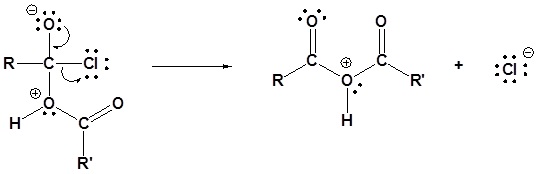
3) Deprotonation
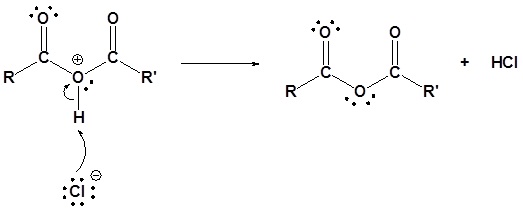
Acid chlorides react with water to form carboxylic acids.
General reaction
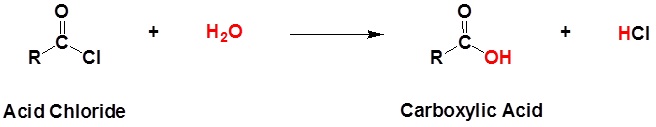
| Example 1: |
|---|
 |
Mechanism
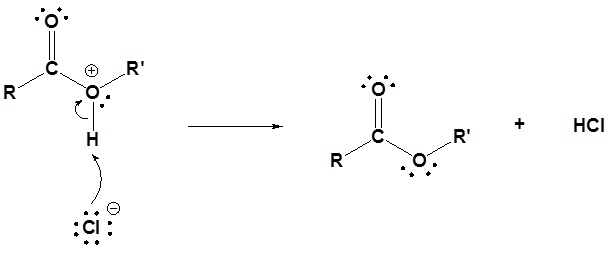
1) Nucleophilic attack by water

2) Leaving group is removed
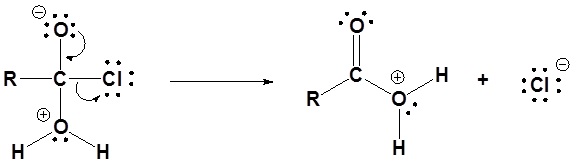
3) Deprotonation
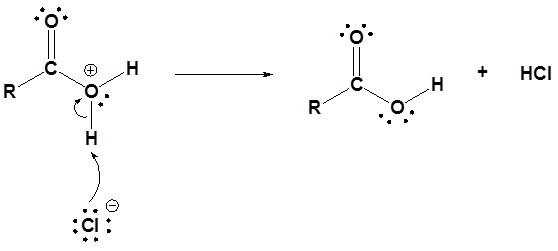
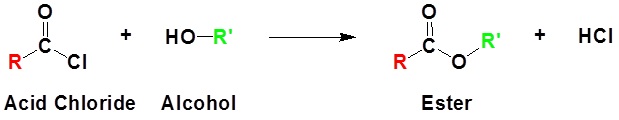
Acid chlorides react with alcohols to form esters
General Reaction
| Example 1: |
|---|
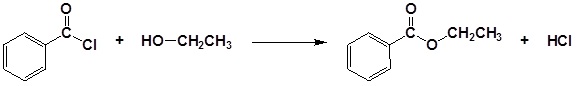 |
Mechanism
1) Nucleophilic attack by the alcohol

2) Leaving group is removed
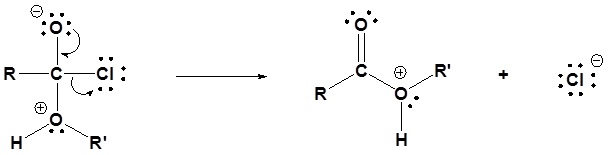
3) Deprotonation
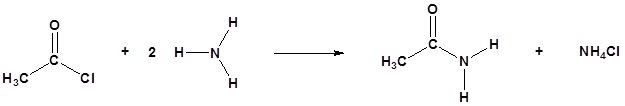
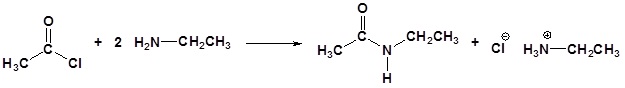
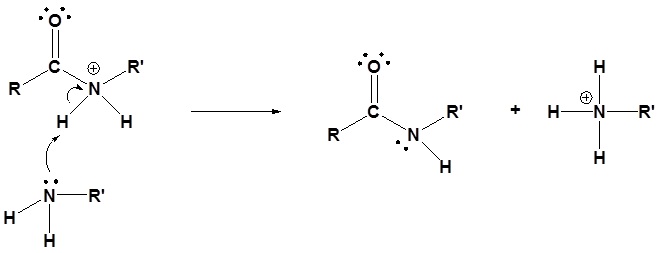
Acid chlorides react with ammonia, 1o amines and 2o amines to form amides.
General Reaction
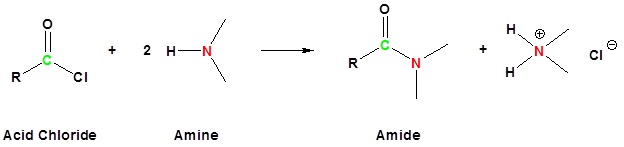
| Examples: |
|---|
|
|
Mechanism
1) Nucleophilic attack by the amine
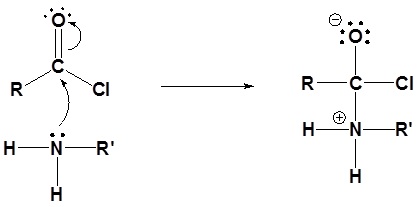
2) Leaving group is removed
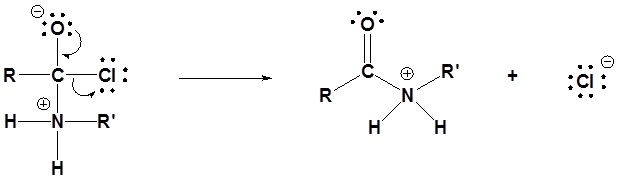
3) Deprotonation
Contributors
Prof. Steven Farmer (Sonoma State University)
Jim Clark (Chemguide.co.uk)