19.8: Synthetic Steroids
- Page ID
- 18337
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into five groups by the receptors to which they bind: glucocorticoids, mineralocorticoids, androgens, estrogens, and progestogens. Vitamin D derivatives are a sixth closely related hormone system with homologous receptors. They have some of the characteristics of true steroids as receptor ligands, but lack the planar fused four ring system of true steroids.
Introduction
Steroid hormones help control metabolism, inflammation, immune functions, salt and water balance, development of sexual characteristics, and the ability to withstand illness and injury. The term steroid describes both hormones produced by the body and artificially produced medications that duplicate the action for the naturally occurring steroids.
A variety of synthetic steroids and sterols have also been contrived. Most are steroids, but some non-steroidal molecules can interact with the steroid receptors because of a similarity of shape. Some synthetic steroids are weaker, and some much stronger, than the natural steroids whose receptors they activate.[6]
Some examples of synthetic steroid hormones:
- Glucocorticoids: alclometasone, prednisone, dexamethasone, triamcinolone
- Mineralocorticoid: fludrocortisone
- Vitamin D: dihydrotachysterol
- Androgens: apoptone, oxandrolone, oxabolone, testosterone, nandrolone (also known as anabolic steroids)
- Oestrogens: diethylstilbestrol (DES)
- Progestins: danazol, norethindrone, medroxyprogesterone acetate, 17-Hydroxyprogesterone caproate.
Some steroid antagonists:
- Androgen: cyproterone acetate
- Progestins: mifepristone, gestrinone
Synthesis
The natural steroid hormones are generally synthesized from cholesterol in the gonads and adrenal glands. These forms of hormones are lipids. They can pass through the cell membrane[5] as they are fat-soluble, and then bind to steroid hormone receptors which may be nuclear or cytosolic depending on the steroid hormone, to bring about changes within the cell. Steroid hormones are generally carried in the blood bound to specific carrier proteins such as sex hormone-binding globulin or corticosteroid-binding globulin. Further conversions and catabolism occurs in the liver, in other "peripheral" tissues, and in the target tissues.
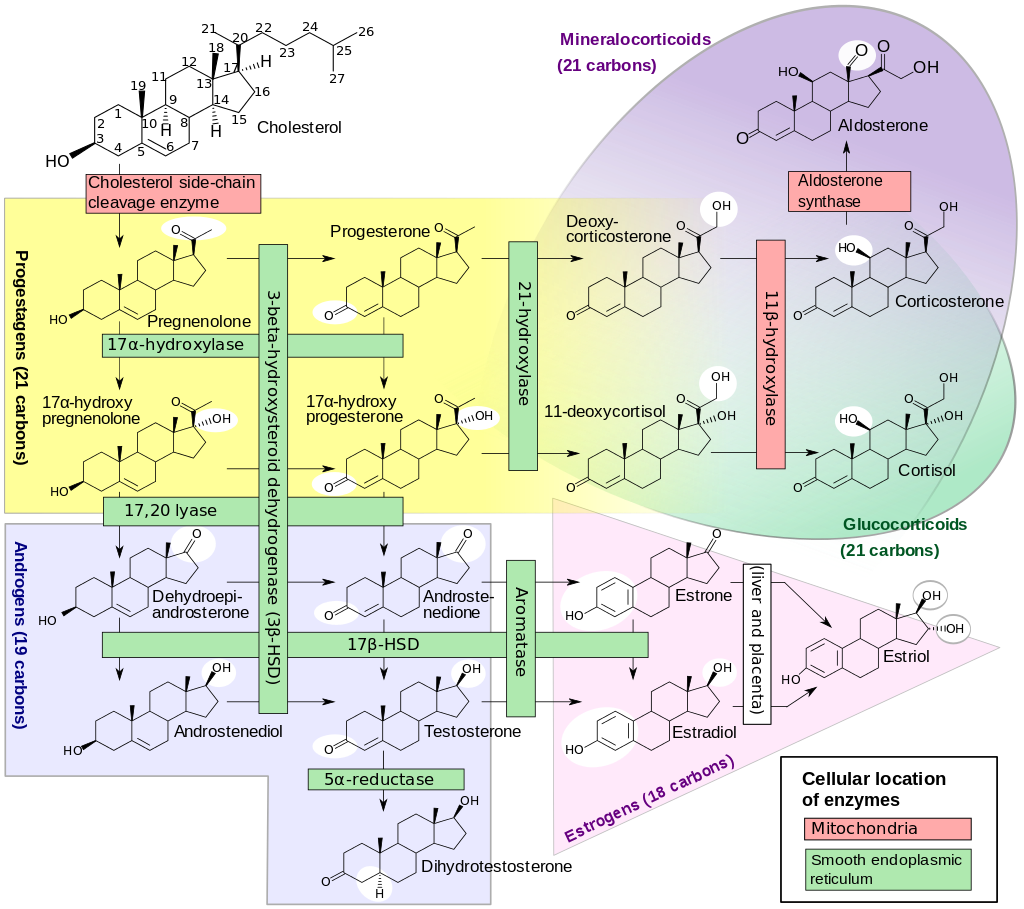
1: Enzymes, their cellular location, substrates and products in human steroidogenesis. Shown also is the major classes of steroid hormones: progestagens, mineralocorticoids, glucocorticoids, androgens and estrogens. However, they partly overlap, e.g. mineralocorticoids and glucocorticoids. White circles indicate changes in molecular structure compared with precursors. For more information on interpretation of molecular structures, see Wikipedia:structural formula. HSD: Hydroxysteroid dehydrogenase References: Walter F. Boron, Emile L. Boulpaep (2003) Medical Physiology: A Cellular And Molecular Approach, Elsevier/Saunders, pp. page 1,300 ISBN: 1-4160-2328-3. Figure used with permission from ).
Contributors
- Wikipedia

