2.3: Step Growth and Chain Growth
- Page ID
- 238851
Step growth and chain growth are two broad classes of polymerization methods. They use monomers with distinct characteristics and display some different growth patterns.
Condensation polymerizations take place via a step-growth process. In a classis condensation reaction, a carboxylic acid reacts with a neutral nucleophile, such as an alcohol or an amine. Substitution leads to formation of either an ester (from an alcohol nucleophile) ar an amide (from an amine nucleophile), with water as a side-product. For example, nylon-6,6 is a condensation polymer.
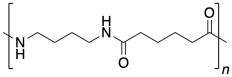
Nylon-6,6 is produced via a condensation reaction between 1,6-hexanedioic acid (or adipic acid) and 1,6-hexanediamine (or hexamethylenediamine). The amine end group of the nucleophile loses a proton and the carboxylic acid loses an OH group, for the formal loss of one water molecule each time an amide bond forms.
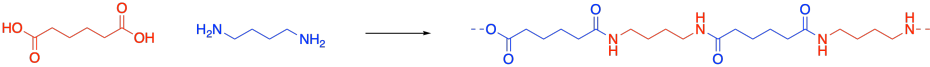
Poly(ethyleneterephthalate) or PETE is a very common example of a polyester.
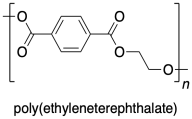
PETE is formed from the reaction of dimethyl terephthalate with 1,2-ethanediol (or ethylene glycol). Once again, a condensation reaction takes place, with a small molecule formed as a side product. An OMe group is displaced fron the carboxyl electrophile and a proton is lost from the nucleophilic alcohol group, adding up to the formal loss of methanol.
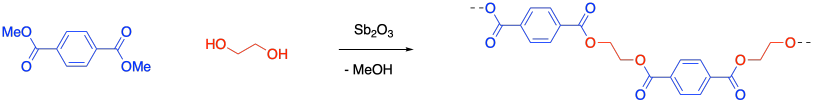
It's worth talking about the conditions used for these reactions. On paper, we might think about using more reactive monomers, such as acyl halide electrophiles, in order to have much faster reactions. However, these reactions are either exothermic in the case of the polyamide or thermoneutral in the case of the polyester, which is formed through a trans-esterification. Both reactions occur in equilibrium. Although they may be relatively slow, they should still get to product eventually, especially if the equilibrium can be shifted to the right. In both of these cases, that shift can be acheived through removal of the water or methanol side-products, which are relatively volatile compared to the other reactants.
Note also that the neutral nucleophile and the relatively poor leaving groups in these cases suggest the reaction will be pretty slow. To speed things up, a Lewis acid may be added (such as the antimony oxide, Sb2O3, employed in the second example).
The monomers used in step-growth polymerization usually have a couple of characteristics that we can see in these examples. Most often, there are two different monomers. Neither monomer would undergo polymerization on its own, but the two monomers are complementary to each other, so that each provides the other with an appropriate reactive partner.
More importantly, each of the monomers we have seen here is mutlifunctional. In this context, functionality describes the number of reactive functional groups. Each of the monomers we have seen so far contains two functional groups; they each have a functionality of two, or they are each difunctional. Once a functional group has reacted on a difunctional monomer, it still has another one that can react. Every monomer can therefore make bonds with two others, allowing for chain formation.
This higher functionality is essential for step-growth polymerization. It isn't limited to difunctional monomers. Monomers could be trifunctional, tetrafunctional, and so on, leading to more highly branched or chemically crosslinked polymers, rather than simple chains.
Problem SM1.1.
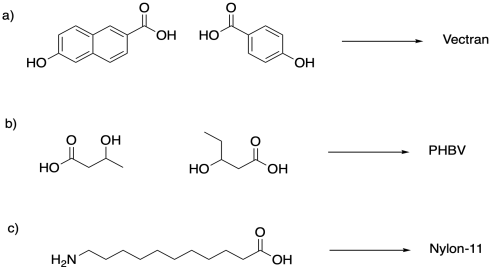
Identify the monomers that would be used to produce these polymers.
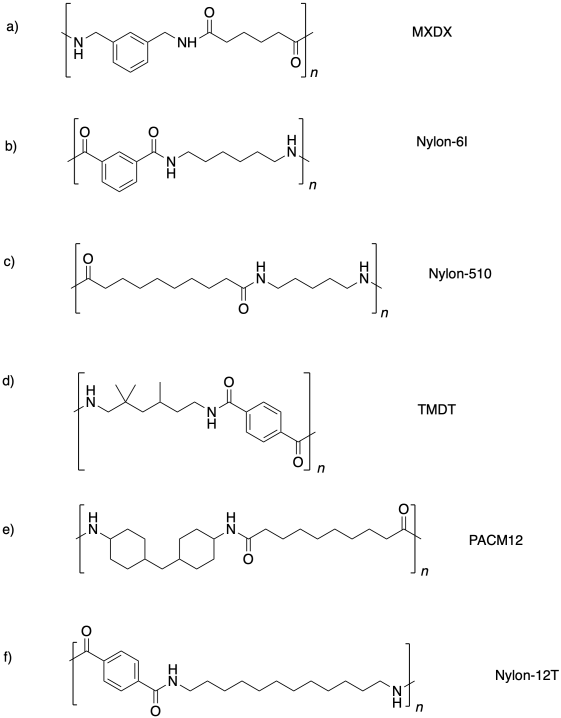
Because of difunctionality in the monomers, condensation polymers can grow in two directions at once. Two complementary monomers connect with each other to form a dimer. Now, suppose all the monomers are reacting at roughly similar rates (not quite true if you know about polymer molecular weight distribution, but close enough). When one end of the dimer goes to react again, chances are it will find another dimer, because most of the monomers have already reacted. That reaction will form a tetramer. The typical tetramer reacting another time will find another tetramer, forming an octamer, and so on.
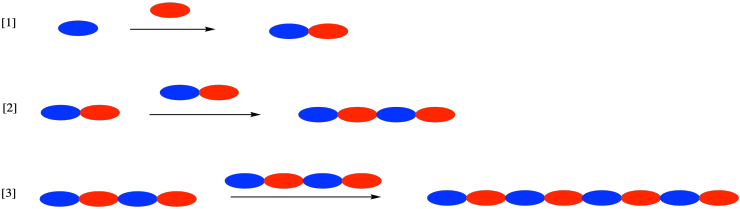
Most of the subsequent chapters in this text deal with a variety of ways of carrying out chain polymerizations. In chain polymerization, we usually start with just one kind of monomer, which is again unreactive by itself. This time, instead of adding a complementary monomer, we add an initiator. The initiator converts one monomer molecule into a reactive intermediate, capable of reacting with another monomer to form a new reactive intermediate, and so on. This is a chain reaction.
Notice the difference from step growth. In chain growth, the polymer chain always grows one monomer at a time. In step growth, the polymer chain doubles with each step. As a result, the rate of growth of the polymer chain is very different in these two cases.
Chain growth results in a steady increase in chain length with every coupling step. Growth is linear.
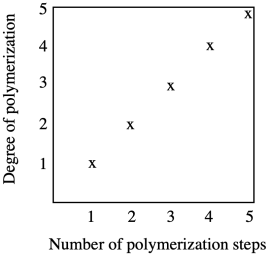
Chain growth is exponential, with chain length doubling at every coupling step. Notice that the y axis on this graph is logarithmic rather than linear.
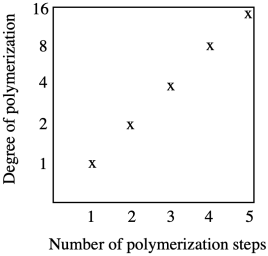
What are the consequences of these two growth patterns? Let's take a look at how many steps it would take to form a high molecular weight polymer, maybe with a molecular weight of one million g/mol (sometimes expressed as one million Da in polymer chemistry; Da stands for Dalton). Starting with a monomer of molecular weight 100 g/mol and using step growth, in which chain length doubles every time a reaction takes place, it will take only thirteen or fourteen steps to reach a molecular weight of a million Da, because 213 = 8,192 repeat units. Using chain growth, it would take ten thousand steps to reach the same molecular weight, assuming a monomer of similar size.
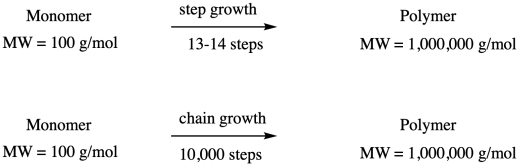
That's a very approximate calculation, and it neglects the weight of side products during condensation polymerization, but we are looking at differences that are orders of magnitude apart.
Why is this difference significant? For one thing, it doesn't matter that much if a condensation reaction is slow. If it only takes twenty (or even thirty) steps to reach a molecular weight of a million (not unheard of in a commercial polymer, but still pretty high), then there's no hurry. In chain polymerization, there is a much higher premium on speed. We need very fast reactions or sometimes very fast catalysts that can turn around and enchain monomer after monomer reliably.
If we take these two broad methods and look at them in a different way, we get new information. If, instead of thinking about the number of bonding events or reaction steps, we focus on the percent conversion, we get a graph that looks like this.
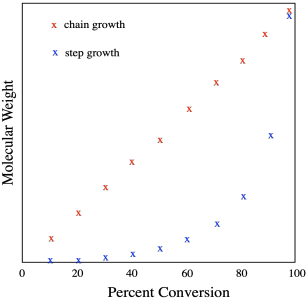
Looked at in this way, we see the linear nature of chain growth. Every time a new monomer is added, the polymer chain grows by the same amount (the weight or length of one new repeat unit, depending on whether we are thiniing in terms of molecular weight or chain length). Step growth works exponentially: doubling, quadrupling, and so on. Both modes of polymerization proceed, ideally, until all of the monomer has completely reacted; in other words, to 100% conversion. But what happens if growth is suddenly arrested because of some unwanted side reaction? In step growth, such an event happening even in the very late stages of polymerization might result in a chain length that is only half what we had expected. Such disastrous results in chain growth would result only if growth stopped after only consuming half the monomer. Arresting chain growth at 80 or 90% conversion still results in pretty long polymer chains, even if they aren't the expected length. Because of this feature, step growth polymerization requires very reliable chemistry. Otherwise, step growth would always result in relatively short chains.
In either case, it's useful to know that polymerization is often followed by a quenching reaction to arrest the process once the polymer has reached the desired chain length. Usually that results in a polymer with two different groups at the end of the chain. The choice of those groups can occasionally be useful in modifying the properties of the polymer.
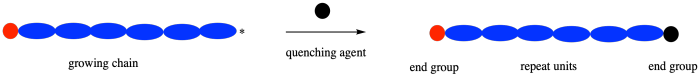
Imagine a case, however, in which this termination happens accidentally. In a chain growth, the polymer has a reactive intermediate at one end only. If something caps that end of the polymer chain, it stops growing altogether. Unexpected terminations can be a significant problems in chain growth.
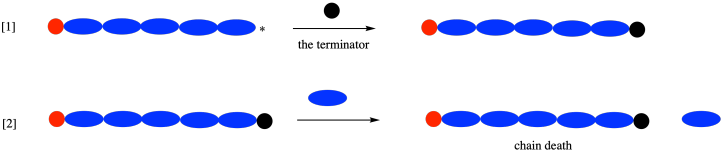
Of course, they are also a problem in step growth, but remember that in step growth the polymer is growing from two ends. If one end gets capped, the other end can keep growing. The polymer will grow more slowly, but it won't stop altogether.
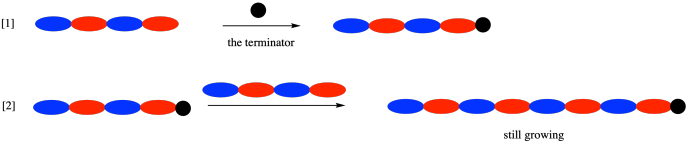
As a result, there is a great deal of interest in developing more reliable ways of carrying out chain growth. That's especially important because one wrong step and chain growth stops altogether. Chain growth is, in fact, a crucial way of making polymers, despite what may seem like some limitations here. A key reason is that step growth is mostly limited to condensation polymerization of carboxyloid compounds such as polyamides and polyesters, as well as a few related materials such as polyurethanes. Chain growth is used to polmerize a wide array of compounds that contain carbob-carbon double bonds (alkenes or olefins) as well as a variety of cyclic monomers. Both approaches are needed to make a range of useful materials.
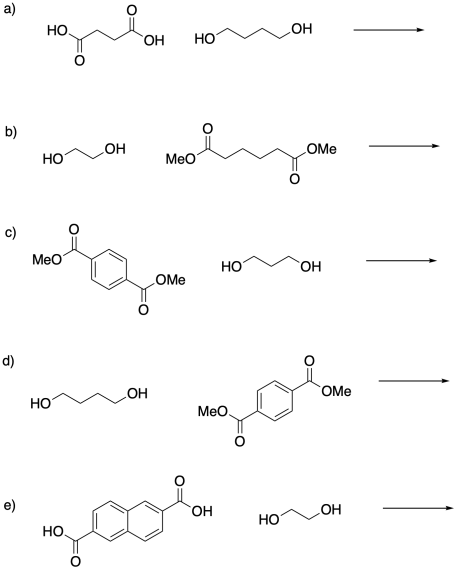
Problem SM1.2.
Provide structures of the polyesters made from the monomers as indicated.
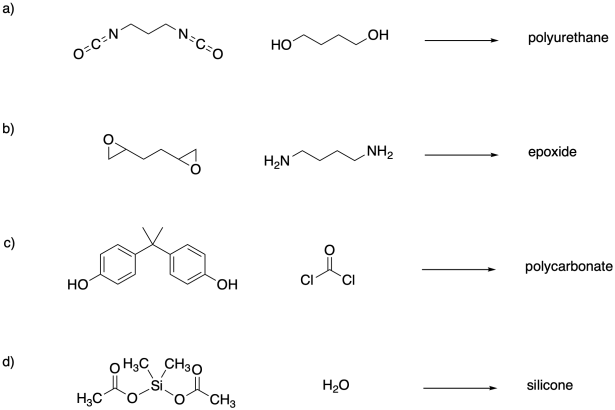
Problem SM1.3.
The following difunctional monomers would undergo polymerization together; in some cases the reaction happens without condensation of a side product. Show the product structures that result.
Problem SM1.4.
A few polymers are obtained from difunctional monomers that contain both nucleophilic and electrophilic sides. Show the polymers that result in these cases.
Problem SM1.5.
Calculate the molecular weight of the polymer in each of the following cases.
a) Step polymerization involving monomers of molecular weight 150 g/mol, allowed to undergo seven polymerization steps.
b) Step polymerization involving monomers of 120 and 130 g/mol, allowed to undergo sixteen polymerization steps.
c) Step polymerization involving monomers of 100 and 105 g/mol, allowed to undergo ten polymerization steps, producing a condensation product with molecular weight 15 g/mol.
d) Chain polymerization of a monomer with molecular weight 105 g/mol, allowed to undergo thirty polymerization steps, using an initiator with molecular weight 65.


