13.1: Prelude to Reactions at the α-Carbon, Part II
- Page ID
- 106371

Introduction
We begin this chapter with the story of two men, and two chemical reactions.
The two men couldn't be more different. One was an acclaimed scientist who lived and continued to work productively into his eighties. The other was struck down as a young boy by what was assumed at the time to be a fatal disease. With the heroic support of his parents and caregivers, though, he lived to his thirtieth birthday and provided the inspiration for development of a medical treatment that could potentially save thousands of lives.
The two chemical reactions in this story are closely related, and both involve the metabolism of fats in the human body. One serves to build up fatty acid chains by repeatedly linking together two-carbon units, while the other does the reverse, progressively breaking off two-carbon pieces from a long chain fatty acid molecule. The life and work of the two men are inextricably linked to the two reactions, and while we will be learning all about the reactions in the main part of this chapter, we'll begin with the stories of the two men.
On a Saturday in January of 2007, Dr. Hugo Moser passed away in the Johns Hopkins Hospital in Baltimore, succumbing to pancreatic cancer. He was 82 years old. A neurologist who had taught and researched for much of his career at Johns Hopkins, he was well known for his workaholic nature: he had signed off on his last grant application while on the way to the hospital for major surgery just a few months previously. Two days after his death, his wife and colleague Ann Moser was back in their lab, because, she said, “He gave us all a mandate to continue with the work”. Dr. Moser was a highly esteemed scientist who had devoted his life to understanding and eventually curing a class of devastating neurodegenerative diseases, most notably adrenoleukodystrophy, or ALD. In his work he was careful, rational, painstaking, and relentless – a classic scientist. But in the minds of many movie fans, he became a Hollywood villain.
Only 17 months after the death of Dr. Moser, newspapers around the world published moving obituaries marking the passing, at age 30, of Lorenzo Odone. In one, written by his older sister and published in the British newspaper The Guardian, Lorenzo as a young boy is described as “lively and charming . . .he displayed a precocious gift for languages as he mastered English, Italian and French. He was funny, articulate and favored opera over nursery rhymes.” But for more than 20 years leading up to his death, he had been confined to a wheelchair, blind, paralyzed, and unable to communicate except by blinking his eyes. Because he was unable to swallow, he needed an attendant to be with him around the clock to suction saliva from his mouth so he wouldn't choke.
When he was he six years old, Lorenzo started to show changes in behavior: a shortening attention span, moodiness. More disturbing to his parents, Augusto and Michaela Odone, was their suspicion that he was having trouble hearing. They took him in to be examined, and although his hearing was fine, the doctors noticed other behavioral symptoms that concerned them, and so ordered more neurological tests. The results were a kick to the stomach: Lorenzo had a fatal neurodegenerative disease called adrenoleukodystrophy. There was no cure; his nervous system would continue to degenerate, and he would probably be dead within two years.
What happened next became such a compelling story that it was eventually retold by director George Miller in the 1992 movie Lorenzo's Oil, starring Nick Nolte and Susan Sarandon as Augusto and Michaela Odone and Peter Ustinov as a character based on Dr. Hugo Moser. The Odones were unwilling to accept the death sentence for their son and, despite having no scientific or medical training, set about to learn everything they could about ALD.
They found out that the cause of ALD is a mutation in a gene that plays an important role in the process by which saturated fatty acids of 26 or more carbons are broken down in the body. When these 'very long chain fatty acids' (VLCFAs) accumulate to excessive levels, they begin to disrupt the structure of the myelin sheath, a protective fatty coating around nerve axons, leading eventually to degradation of the nervous system.
Researchers had found that restricting dietary intake of VLCFAs did not help – apparently much of the damage is done by the fats that are naturally synthesized by the body from shorter precursors. The Odones realized that the key to preventing destruction of the myelin sheath might be to somehow disrupt the synthesis of VLCFAs in Lorenzo's cells. The breakthrough came when they came across studies showing that the carbon chain-elongating enzyme responsible for producing VLCFAs is inhibited by oleic and erucic acids, which are monounsaturated fatty acids of 18 and 22 carbons, respectively and are found in vegetable oils.
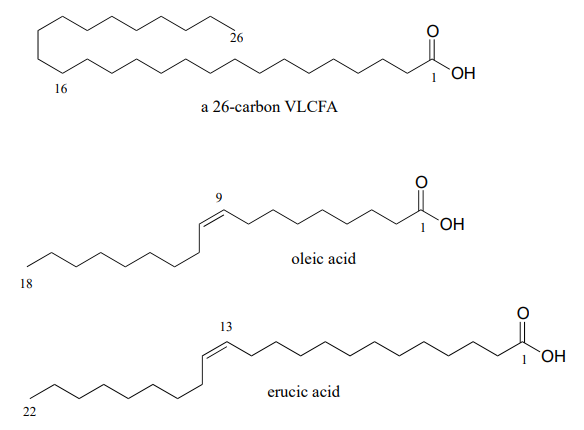
Administration of a mixture of these two oils, which eventually came to be known as 'Lorenzo's Oil' , was shown to lead to a marked decrease in levels of VLCFAs in ALD patients.
This was, however, a therapy rather than a miracle cure – and tragically for the Odones and the families of other children afflicted with ALD, the oil did not do anything to reverse the neurological damage that had already taken place in Lorenzo's brain. Although he was profoundly disabled, with round-the-clock care and a daily dosage of the oil Lorenzo was able to live until a day after his 30th birthday, 22 years longer than his doctors had predicted.
The story does not end there. Although the discovery of the treatment that bears his name came too late for Lorenzo Odone, might daily consumption of the oil by young children who are at a high genetic risk for ALD possibly prevent onset of the disease in the first place, allowing them to live normal lives? This proposal was not without a lot of controversy. Many ALD experts were very skeptical of the Lorenzo's oil treatment as there was no rigorous scientific evidence for its therapeutic effectiveness, and indeed erucic oil was thought to be potentially toxic in the quantities ingested by Lorenzo. Most doctors declined to prescribe the oil for their ALD patients until more studies could be carried out. The Hollywood version of Lorenzo's story cast the medical and scientific establishment, and Dr. Hugo Moser in particular, in a strikingly negative light – they were portrayed as rigid, callous technocrats who cared more about money and academic prestige than the lives of real people. Dr. Moser was not mentioned by name in the movie, but the character played by Peter Ustinov was based closely on him: as his obituary in the Washington Post recounts, Dr. Moser once told an interviewer "The good guys were given real names. The bad guys were given pseudonyms."
What Hugo Moser in fact did was what a good scientist should always do: he kept an open mind, set up and performed careful, rigorous experiments, and looked at what the evidence told him. In a 2005 paper, Moser was finally able to confidently report his results: when young children at risk of developing ALD were given a daily dose of Lorenzo's oil, they had significantly better chance of avoiding the disease later on.
When he died, Dr. Moser was tantalizingly close to demonstrating conclusively that a simple and rapid blood test that he and his team had developed could reliably identify newborns at high risk of developing ALD – but it was not until after his death that his colleagues, including his wife, Ann Moser, were able to publish results showing that the test worked. The hope is that many lives might be saved by routinely screening newborns for ALD and responding with appropriate preventive treatments - possibly including Lorenzo's oil.
The biochemical reactions at the heart of the Lorenzo's oil story – the carbon-carbon bond forming and bond breaking steps in the synthesis and degradation of fatty acids - both involve chemistry at the \(\alpha\)-carbon and proceed through enolate intermediates, much like the aldol and isomerization reactions we studied in chapter 11. They are known as 'Claisen condensation' and 'retro-Claisen cleavage' reactions, respectively, and represent another basic mechanistic pattern - in addition to the aldol reaction - that is ubiquitous in metabolism as a means of forming or breaking carbon-carbon bonds.
To begin this chapter, we will first learn about 'carboxylation' and 'decarboxylation' reactions, in which organic molecules gain or lose a bond to carbon dioxide, respectively, in a mechanism that is really just an extension of the aldol/retro-aldol reactions we learned about in the previous chapter. As part of this discussion, we will work through the mechanism of the carbon-fixing enzyme in plants commonly known as 'Rubisco', which is thought to be the most abundant enzyme on the planet. Then, we will move to the Claisen reactions that are so central to lipid metabolism and the story of Lorenzo Odone. Finally, we will study 'conjugate additions' and '\(\beta\)-eliminations', common reaction patterns that involve double bonds in the \(\alpha-\beta\) position relative to a carbonyl group, and which, again, proceed via enolate intermediates.


