12.2: Review of Acidity at the α-Carbon
- Page ID
- 106365
Let's review what we learned in section 7.6 about the acidity of a proton on an a-carbon and the structure of the relevant conjugate base, the enolate ion. Remember that this acidity can be explained by the fact that the negative charge on the enolate conjugate base is delocalized by resonance to both the \(\alpha\)-carbon and the carbonyl oxygen.
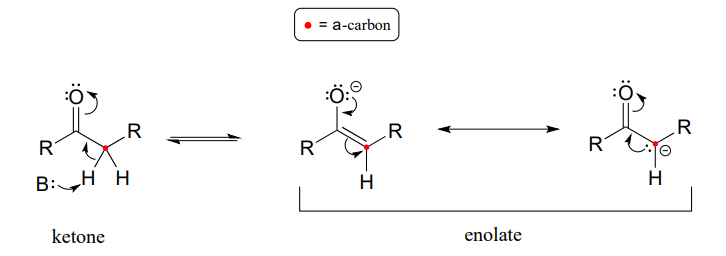
The \(\alpha\)-carbon on the enolate is \(sp^2\)-hybridized with trigonal planar geometry, as are the carbonyl carbon and oxygen atoms (now would be a good time to go back to section 2.1, section 2.2, and section 2.3 to review, if necessary, the geometry of p-bonding in conjugated systems). \(pK_a\) of a typical a-proton in aqueous solution is approximately 18-20: acidic, but only weakly so. Recall from section 7.8, however, that the effective \(pK_a\) of a functional group on an enzyme-bound molecule can be altered dramatically by the 'microenvironment' of the active site. In order to lower the \(pK_a\) of an \(\alpha\)-proton, an enzyme catalyzing a reaction that begins with an a-proton abstraction step must further stabilize the negative charge that develops on the oxygen atom of the (enolate) conjugate base. Different enzymes have evolved different strategies for accomplishing this task: in some cases, a metal cation (often \(Zn^{+2}\)) is bound in the active site to provide a stabilizing ion-ion interaction. In other cases, stabilization is provided by a proton-donating group positioned near the oxygen. As a third possibility, the active site architecture sometimes provides one or more stabilizing hydrogen bond donor groups.
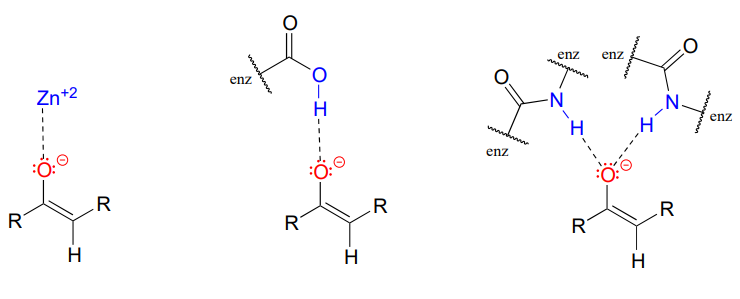
In most of the mechanism illustrations in this chapter where an enolate intermediate is depicted, stabilizing metal ions or hydrogen bond interactions will not be explicitly drawn, for the sake of clarity. However, whenever you see an enolate intermediate in an enzyme-catalyzed reaction, you should remember that there are stabilizing interactions in play inside the active site.


