11.8: Protein Synthesis on the Ribosome
- Page ID
- 106594
Recall from section 1.3D that the 'peptide bonds' which link amino acids to form polypeptides and proteins are in fact amide functional groups. The figure below shows the first four amino acid residues in a protein, starting at the amino terminus.
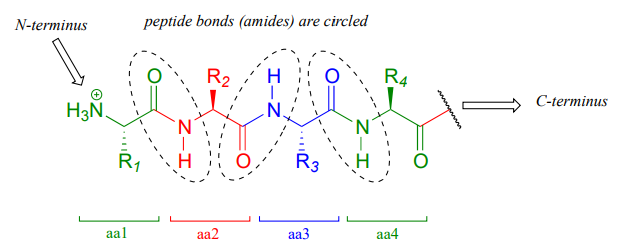
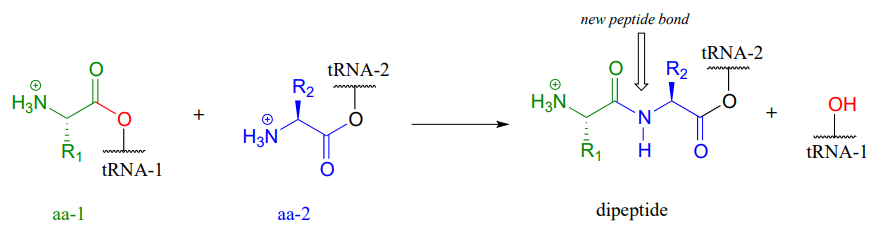
Let’s take a look at the chemistry behind the formation of a new peptide bond between the first two amino acids - which we will call \(aa-1\) and \(aa-2\) - in a growing protein molecule. This process takes place on the ribosome, which is essentially a large biochemical 'factory' in the cell, composed up of many enzymes and \(RNA\) molecules, and dedicated to the assembly of proteins. You will learn more in a biochemistry or cell biology course about the complex but fascinating process of ribosomal protein synthesis. For now, we will concentrate on the enzyme-catalyzed organic transformation that is taking place: the formation of an amide from a carboxylate and an amine.
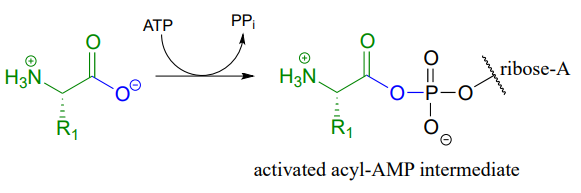
We have seen amide-forming reactions before – think back to the glutamine and asparagine synthetase reactions (section 11.5). The same ideas that we learned for those reactions hold true for peptide bond formation: the carboxylate group on a substrate amino acid must first be activated, and the energy for this activation comes from ATP.
The carboxylate group of aa-1 is first transformed to an acyl-AMP intermediate through a nucleophilic substitution reaction at the \(\alpha \)-phosphate of ATP.
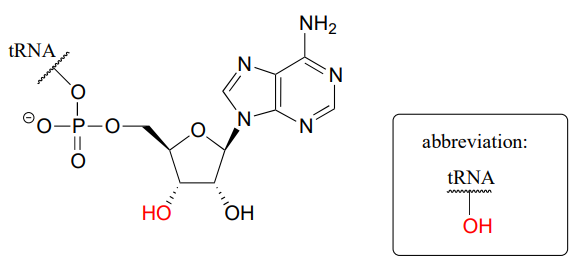
In the next step, the amino acid is transferred to a special kind of \(RNA\) polymer called transfer \(RNA\), or \(tRNA\) for short. We need not concern ourselves here with the structure of \(tRNA\) molecules- all we need to know for now is that the nucleophile in this reaction is a hydroxyl group on the terminal adenosine of a \(tRNA\) molecule. Because this \(tRNA\) molecule is specific to \(aa-1\), we will call it \(tRNA-1\)
The incoming nucleophile is an alcohol, thus what we are seeing is an esterification: an acyl substitution reaction between the activated carboxylate of \(aa-1\) and an alcohol on \(tRNA-1\) to form an ester.
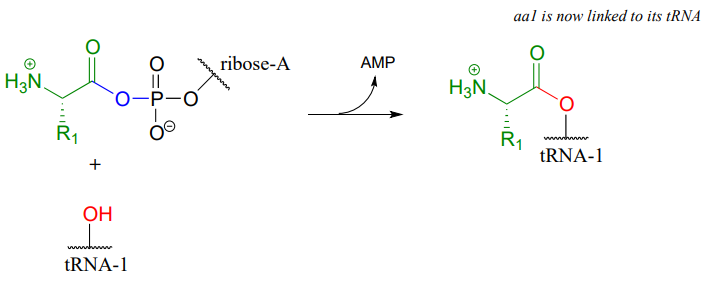
This reaction, starting with activation of the amino acid, is catalyzed by a class of enzymes called aminoacyl-\(tRNA\) synthetases (there are many such enzymes in the cell, each one recognizing its own amino acid - \(tRNA\) pair).
The first amino acid is now linked via an ester group to \(tRNA-1\). The actual peptide bond-forming reaction occurs when a second amino acid (aa-2) also linked to its own \(tRNA-2\) molecule, is positioned next to the first amino acid on the ribosome. In another acyl substitution reaction, catalyzed by an enzymatic component of the ribosome called peptidyl transferase (EC 2.3.2.12), the amino group on \(aa-2\) displaces \(tRNA1\): thus, an ester has been converted to an amide (thermodynamically downhill, so ATP is not required).
This process continues on the ribosome, as one amino acid after another is added to the growing protein chain:

When a genetically-coded signal indicates that the chain is complete, an ester hydrolysis reaction – as opposed to another amide formation - occurs on the last amino acid, which we will call \(aa-n\). This reaction is catalyzed by proteins called release factors (RFs).
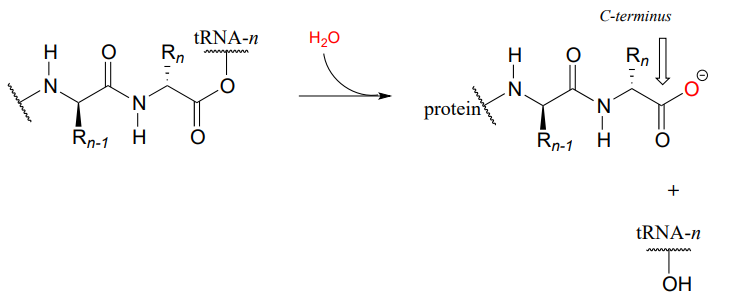
This hydrolysis event frees the mature protein from the ribosome, and results in the formation of a free carboxylate group at the end of the protein (this is called the carboxy-terminus, or \(C\)-terminus of the protein, while the other end – the ‘starting’ end – is called the \(N\)-terminus).


