11.1: Prelude to Nucleophilic Acyl Substitution Reactions
- Page ID
- 106357

Introduction
The 26th of July, Notice is given to the Sheriffs, that in the Street of Lescalle, a Part of the old Town inhabited only by poor People, Fifteen Persons are suddenly fallen sick: They dispatch thither Physicians and Surgeons; they examine into the Distemper, and make Report; some, that 'tis a Malignant Fever; others, a contagious or pestilential Fever, occasioned by bad Food, which Want had long forced those poor Creatures to live upon . . .
The 27th, Eight of those Sick dye; the Sheriffs themselves go to their Houses to cause them to be searched; Buboes [swelling of the lymph nodes] are found on Two of them: The Physicians and Surgeons still hold the same Language, and impute the Cause of the Distemper to unwholsome Food. Notwithstanding which, as soon as Night comes, M. Moustier repairs to the Place, sends for Servants from the Infirmaries, makes them willingly or by Force, take up the Bodies, with all due Precautions; they are carried to the Infirmaries, where they are buried with Lime; and all the rest of the Night he causes the remaining Sick, and all those of their Houses, to be removed to the Infirmaries.
The 28th, very early in the Morning, Search is made every where for those who had Communication with them, in order to confine them: Other Persons in the same Street fall sick, and some of those who first sicken'd dye. ..
The People who love to deceive themselves, and will have it absolutely not to be the Plague, urge a Hundred false Reasons on that Side. Would the Plague, say they, attack none but such poor People? Would it operate so slowly?
Let them have but a few Days Patience, and they will see all attacked without Distinction, with the swiftest Rage, and the most dreadful Havock, that ever was heard of.
(source: Gutenberg Project http://www.gutenberg.org/files/45673...-h/45673-h.htm)
In late May of 1720, a ship arrived in the Mediterranean port city of Marseille, having recently departed from Cyprus and Tripoli. Although several crew members had fallen ill and died during the journey, the ship was allowed to unload after only a very brief quarantine, the result of political pressure on port authorities from local businessmen who wanted quick access to the valuable silk and cotton waiting in the ship's hold.
Along with silk and cotton, the hold carried rats. The rats, in turn, carried fleas. The fleas carried a microscopic mass murderer: Yersinia pestis, the species of bacteria that causes bubonic plague.
It is next to impossible to estimate how many people have died from bubonic plague over the course of human history. In the time of the 'Black Death' in the 14th century, it wiped out more than half the population of Europe. In the Great Plague of Marseille in 1720, over 100,000 people succumbed to Y. pestis infection in the city and surrounding provinces. At the height of the outbreak, corpses piled up in city streets, and a fortified wall, the 'mur de la peste' was constructed in an attempt to prevent people from traveling north to the neighboring city of Aix.
Throughout history, bacteria have been the cause of untold human death and suffering, making the threat posed by more obviously frightening species - lions and bears, spiders and snakes – seem inconsequential by comparison. As recently as the mid-1940s, a minor cut or cold could become a life-threatening event if a bacterial infection were to set in, and even in developed countries, one in twenty infants did not survive to celebrate their first birthday.
Since then, the infant mortality rate in developed countries has declined by a factor of ten. You probably don't worry very much when a small cut on your hand becomes infected. The idea of half of the population of the United States dying in a plague is, in most people's minds, the stuff of zombie movies, not reality. Bacteria are, for now at least, no longer public enemy #1.
How did this happen?
For an answer, we move to a September morning in 1928, in the laboratory of Alexander Fleming, a Scottish bacteriologist working at St. Mary's Hospital in London. As a young man serving in the British Medical Corps during World War I, Fleming saw first-hand how deadly bacteria could be, as he watched countless soldiers in his battlefield hospital die from infected wounds. After returning to civilian life, he began to study Staphylococci bacteria, a common source of life-threatening infections in humans, hoping to discover new antibacterial agents that were more effective than those he had used in the war. He spent a lot of his time growing Staphylococcus cultures in petri dishes for his experiments, and, notoriously untidy, he tended to leave piles of culture dishes lying around his lab. One morning, he returned from a short vacation to find that one of the cultures he had left out had some mold growing on it. He was about to throw it away, but happened to notice something curious: surrounding the small spot of mold was a circle of clear medium, where no bacteria were growing. He realized that the mold must be secreting something that killed bacteria.
As it turned out, the mold was a of strain called Penicillium notatum, and the 'something' killing the bacteria was an organic compound that came to be known as penicillin.
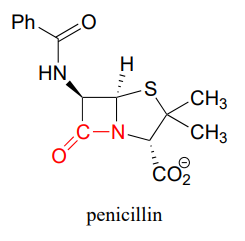
Fleming published his findings in the British Journal of Experimental Pathology, but made only passing reference to the potential therapeutic value of penicillin. The paper received little attention.
Fast-forward now to early February 1941, with the world once again at war. One morning, a policeman named Albert Alexander living in Oxford, England, had an unfortunate gardening accident. While he was trimming some roses on his day off, his shears slipped and gave him a nasty cut on the side of his mouth. The cut became infected, and after a few days it appeared as if the infection would kill him. Then, he got a visit in his hospital room from some chemists at nearby Oxford University.
For the last few years, the chemists had been hard at work isolating pure penicillin from mold cultures, a tricky job because the compound tends to degrade during purification. It is a feat that Alexander Fleming -who, after all, was a bacteriologist, not a chemist - had never been able to accomplish, but the Oxford researchers had realized how valuable penicillin might be to the war effort, and had finally met with some success. They needed a human subject on whom to test the ability of their compound to treat infected wounds, and Albert was their man for the job. They injected him with penicillin, and within a day his infection cleared up. It was a new day in the history of medicine.
At the heart of a penicillin molecule is an amide functional group - more specifically, a cyclic amide, or 'lactam'. To understand how penicillin works at the molecular level as it prevents bacteria from multiplying, we first need to know more about the chemistry of amides and other carboxylic acid derivative functional groups, and a type of organic reaction mechanism called 'nucleophilic acyl substitution'. Understanding the reactivity of carboxylic acid derivative groups will also allow us to appreciate why penicillin is so prone to degradation, and why - very significantly for all of us - the era of not having to worry about bacterial infections may be near an end, as common toxic bacterial species such as Staphylococcus develop increasingly robust resistance to antibiotics.


