Solutions to Chapter 2 exercises
- Page ID
- 80303
E2.1:
E2.2: sp3 orbital on carbon overlapping with 3p orbital on chlorine.
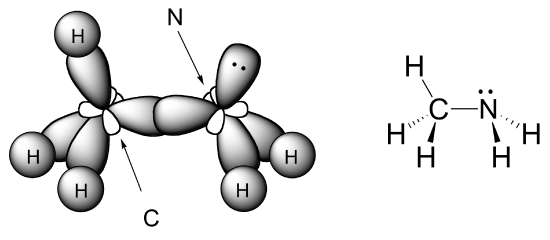
E2.3: Both the carbon and the nitrogen atom in CH3NH2 are sp3-hybridized. The C-N sigma bond is an overlap between two sp3 orbitals.
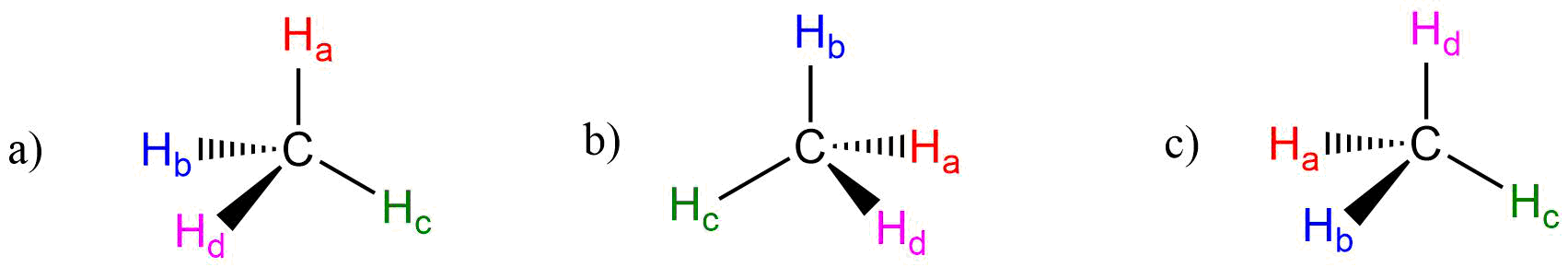
E2.4: Atoms in red all lie in the same plane.
E2.5: The carbon atoms in an aromatic ring are sp2 hybridized, thus bonding geometry is trigonal planar: in other words, the bonds coming out of the ring are in the same plane as the ring, not pointing above the plane of the ring as the wedges in the incorrect drawing indicate. A correct drawing should use lines to indicate that the bonds are in the same plane as the ring:

E2.6:
a) The carbon and nitrogen atoms are both sp2 hybridized. The carbon-nitrogen double bond is composed of a sigma bond formed from two sp2 orbitals, and a pi bond formed from the side-by-side overlap of two unhybridized 2p orbitals.
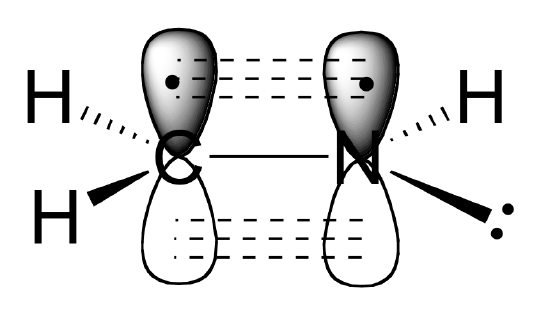
b) As shown in the figure above, the nitrogen lone pair electrons occupy one of the three sp2 hybrid orbitals.
fig 4
E2.7:
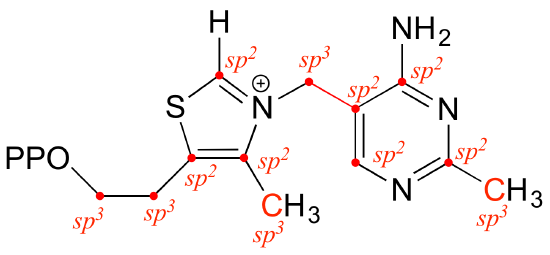
E2.8:
a)
bond b: Nsp2-Csp3 (this means an overlap of an sp2 orbital on N and an sp3 orbital on C)
bond c: Csp2-Csp2 plus C2p-C2p (pi)
bond d: Csp2-Csp3
bond e: Csp3-Csp3
bond f: Csp3-Csp3
bond g: Csp2-Csp2 (s) plus C2p-C2p (pi)
bond h: Csp2-H1s
bond i: Csp2-Csp2
b)
bond a: lone pair on N occupies an sp2 orbital
bond e: lone pair on N occupies an sp3 orbital
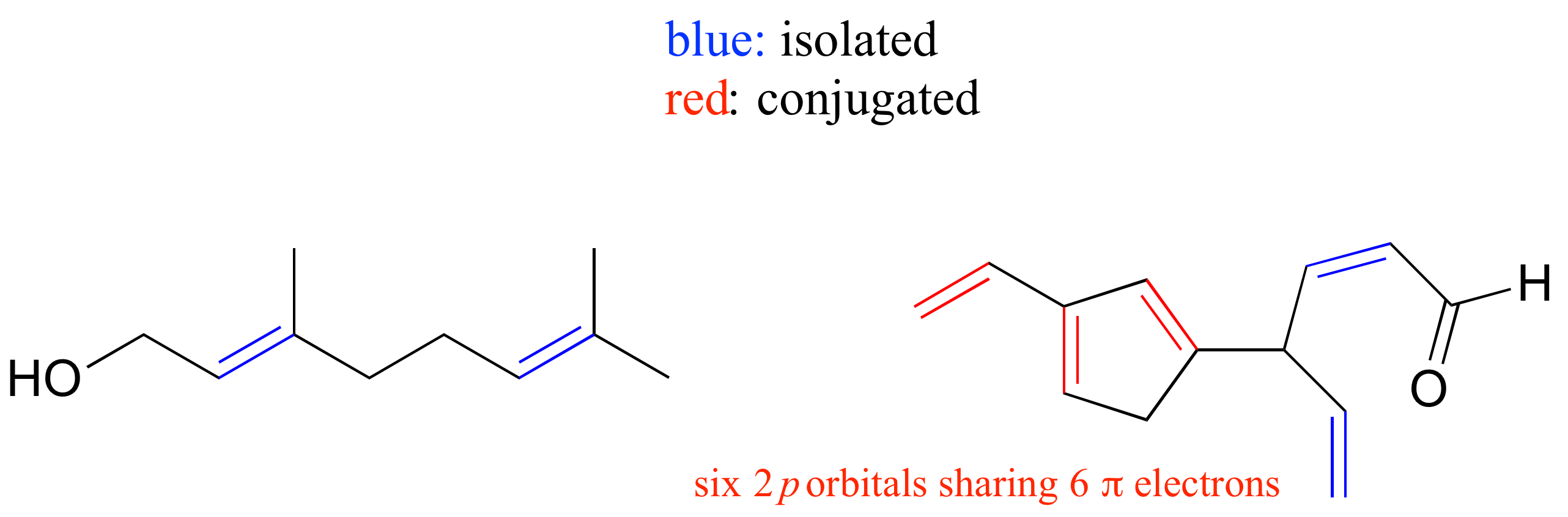
E2.9:
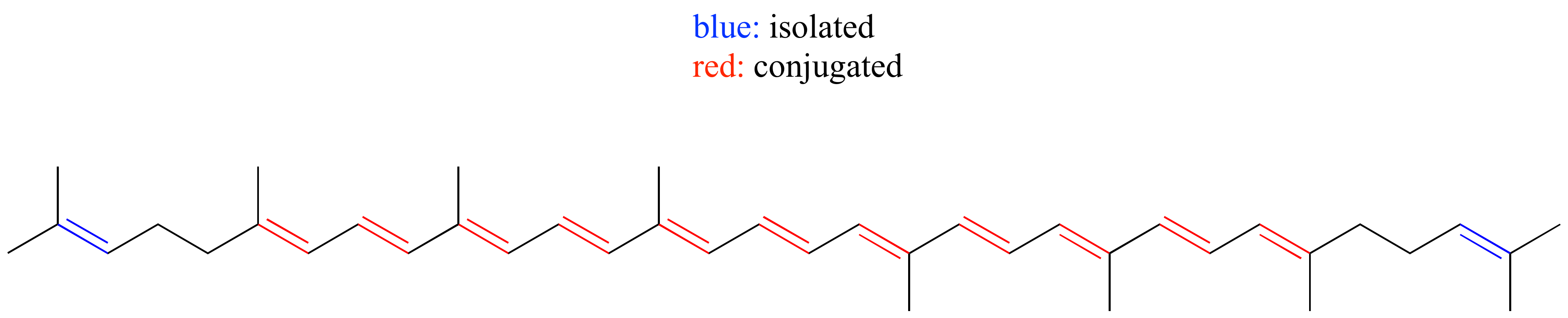
E2.10: The conjugated system contains 22 2p orbitals sharing 22 pi electrons
E2.11:
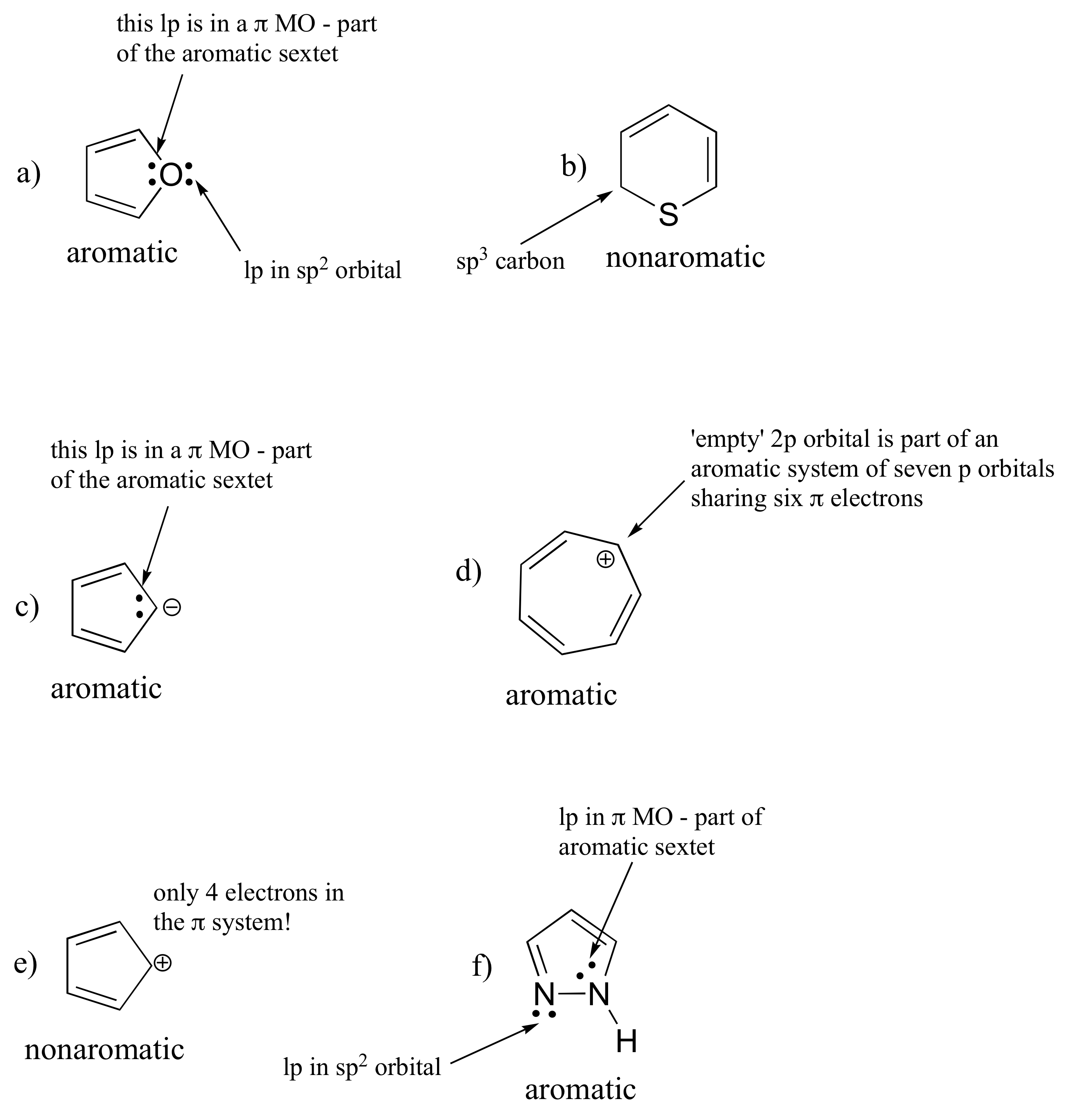
Indole: nitrogen is 'pyrrole-like' (lone pair is part of aromatic ring)
Purine: N1, N3, and N7 are pyridine-like (lone pair in sp2 orbital); N9 is 'pyrrole-like'.
E2.12:
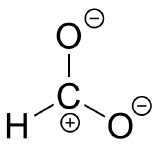
E2.13:
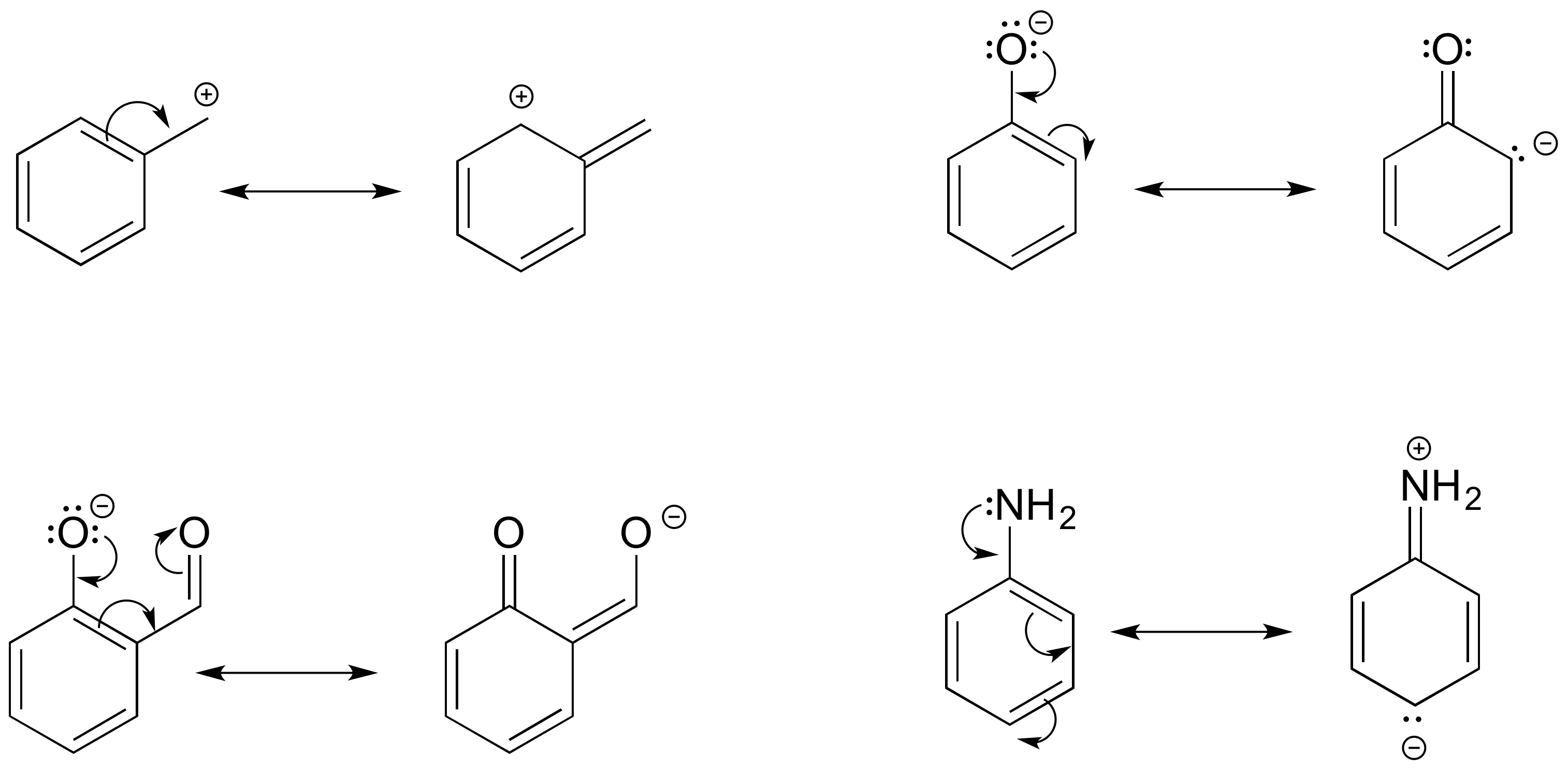
E2.15:
E2.16:
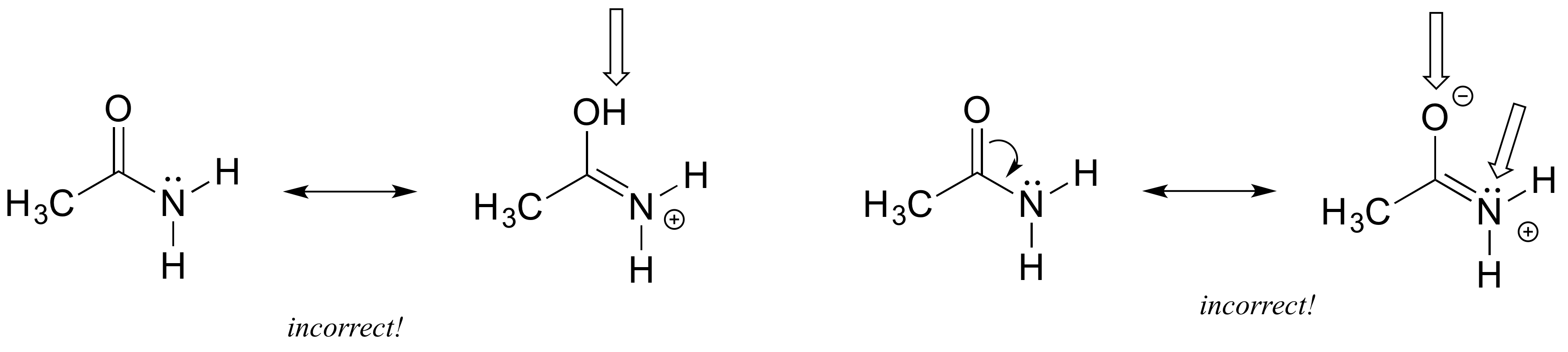
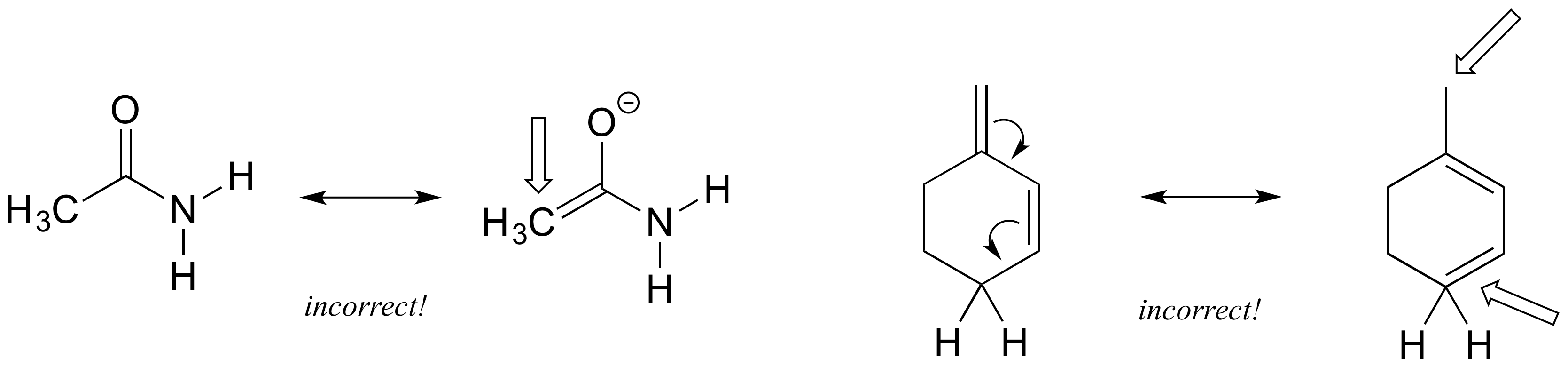
Left: an H atom has been added - in resonance contributors, only pi electrons and lone pairs are rearranged.
Right: Following the curved arrow, the oxygen atom should have only 6 electrons and thus a positive formal charge. Also the nitrogen is breaking the octet rule (remember that drawing lone pairs is optional, so even if they are not drawn you need to assume they are there).
Left: The CH3 carbon is breaking the octet rule with 5 bonds.
Right: One carbon would have a positive formal charge if the arrows are followed, and the other breaks the octet rule with 5 bonds (keep careful track of hydrogen atoms when they are not drawn in line structures!)
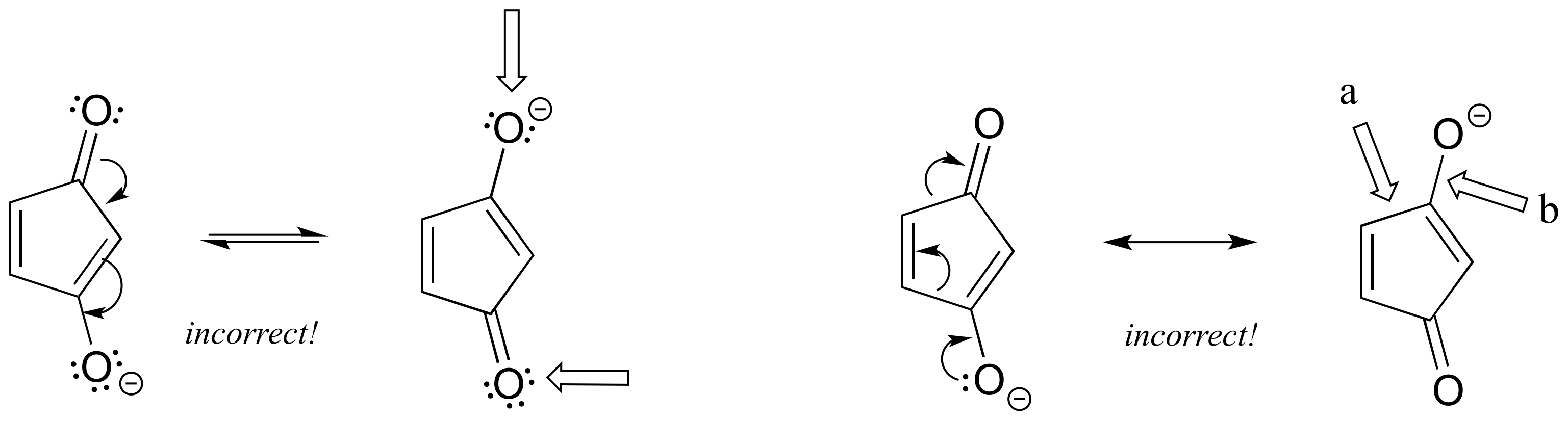
Left: One oxygen should have a positive formal charge, and one breaks the octet rule.
Right: a) the arrow shows this single bond breaking - you can't break single bonds in a resonance contributor. b) the arrow shows a triple bond forming here, which would also mean the oxygen is breaking the octet rule.
E2.17:
a)
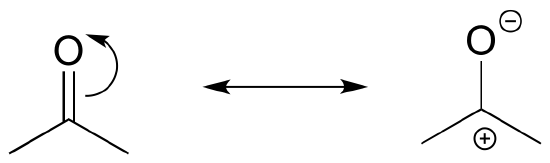
The contributor on the left is minor because it a) has a separation of charges, and b) the carbon has an incomplete octet.
b) Acetone and 2-propanol have the same molecular formula but different atom-to-atom bonding arrangements. Therefore, they are constitutional isomers, not resonance contributors.
E2.18: Minor contributors have additional separation of charge.
E2.19:
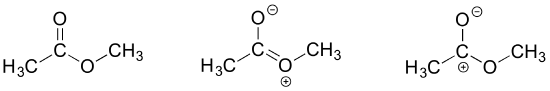
The contributor on the left is the most stable: there are no formal charges.
The contributor on the right is least stable: there are formal charges, and a carbon has an incomplete octet.
The contributor in the middle is intermediate stability: there are formal charges, but all atoms have a complete octet.
E2.20: Consult your instructor or tutor for an evaluation of your orbital drawings. Both contributors should show three overlapping p orbitals (on the oxygen, carbonyl carbon, and nitrogen) sharing four pi electrons.
E2.21: Consult your instructor or tutor for an evaluation of your orbital drawings. Both contributors should show three overlapping p orbitals sharing four pi electrons.
E2.22: This contributor is major because there are no formal charges.
E2.23:
a)
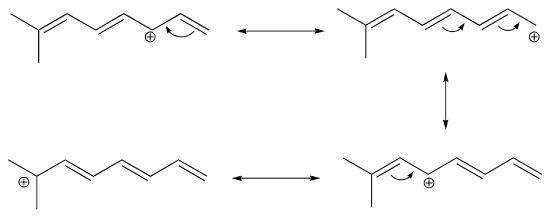
b) The conjugated pi system in this carbocation is composed of seven 2p orbitals containing six delocalized pi electrons.
E2.24:
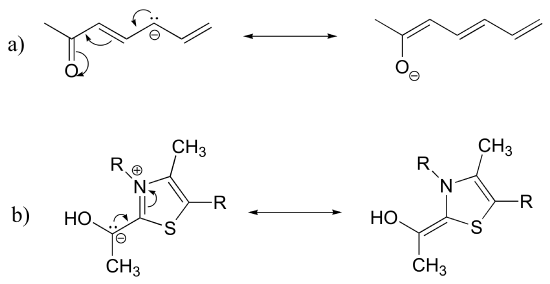
c) The conjugated pi system in structure a) is composed of seven 2p orbitals containing eight delocalized pi electrons.
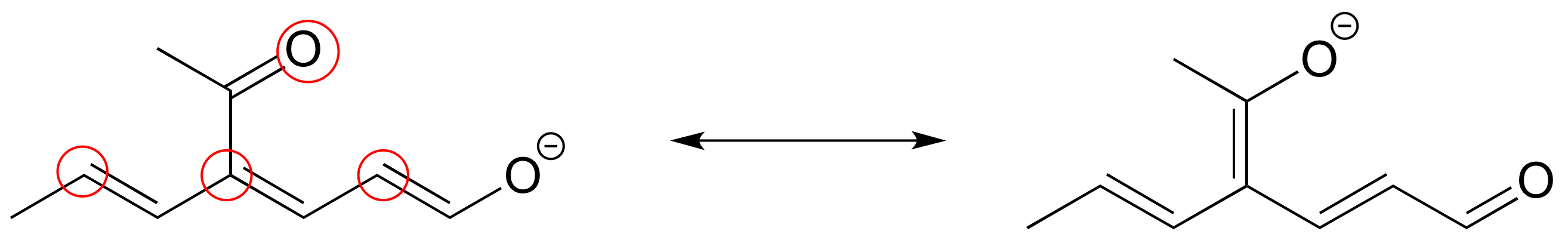
E2.25: The two major contributors or those in which the negative formal charge is located on an oxygen rather than on a carbon.
E2.26: The horizontal trend is based on atomic number (the number of protons in the nucleus). For example, fluorine is more electronegative than carbon, because the fluorine nucleus contains three more protons, the positive charges on which pull negatively-charged electrons closer to the nucleus.
The vertical trend is based on atom size, specifically the size of the 'electron cloud' surrounding the nucleus. For example, fluorine is more electronegative than chlorine (even though chlorine contains more protons) because the outermost valence electrons on fluorine, which are in the n = 2 "shell", are closer to the nucleus than the valence electrons in chlorine, which occupy the n = 3 "shell". The fluorine electron cloud, therefore, is subject to greater electrostatic attractive forces from protons (electrostatic forces decrease rapidly as the distance between the positive and negative charges increases.)
E2.27: Only molecule (b) does not have a molecular dipole, due to its symmetry (bond dipoles are equal and in opposite directions).
E2.28: To be a hydrogen bond donor, the molecule needs to have a hydrogen bound to N, O, or F. To be an acceptor, it merely needs an N, O, or F.
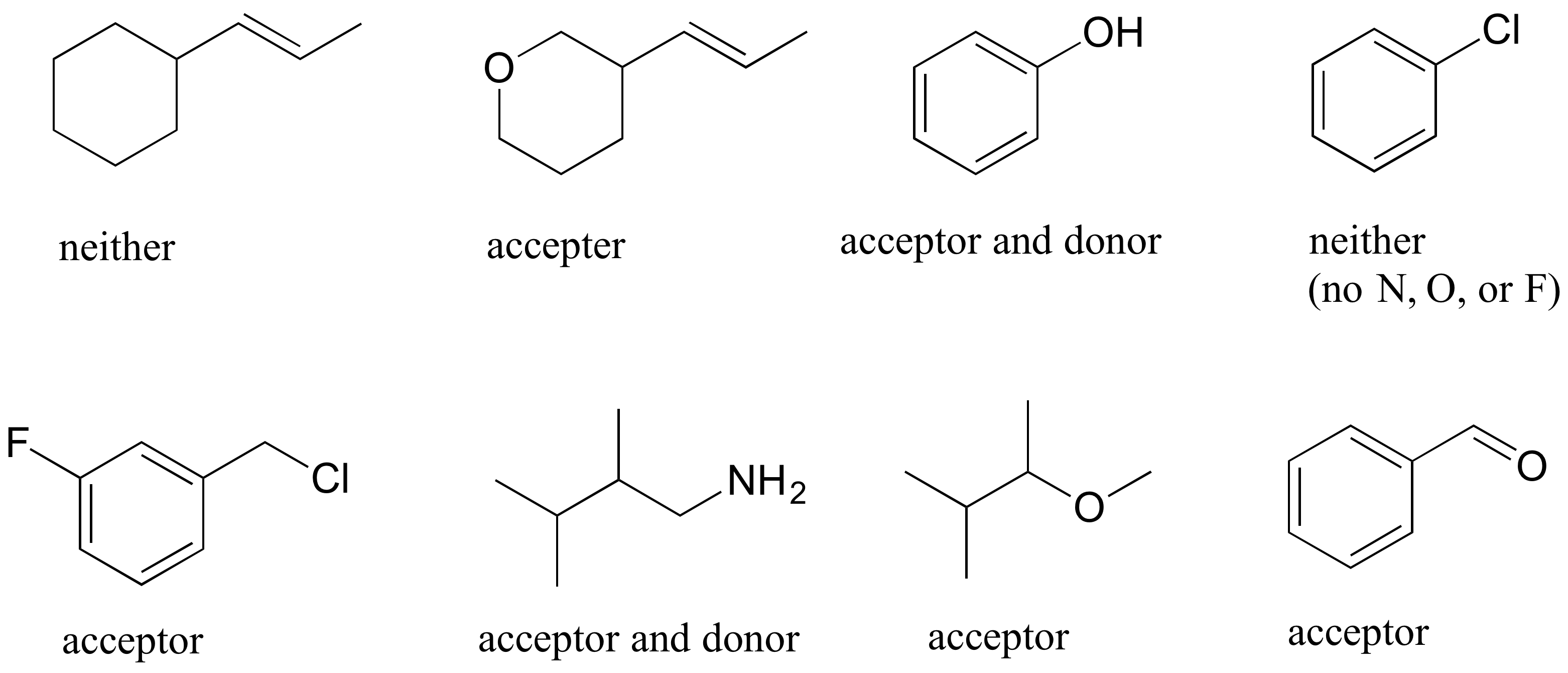
E2.29: Note in part (c) that methyl acetate can only be a hydrogen bond acceptor, not a donor.
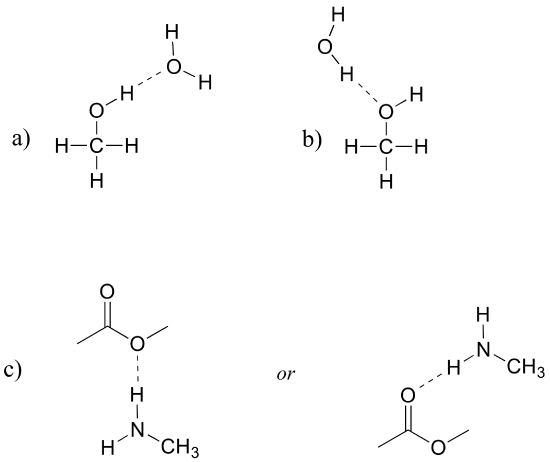
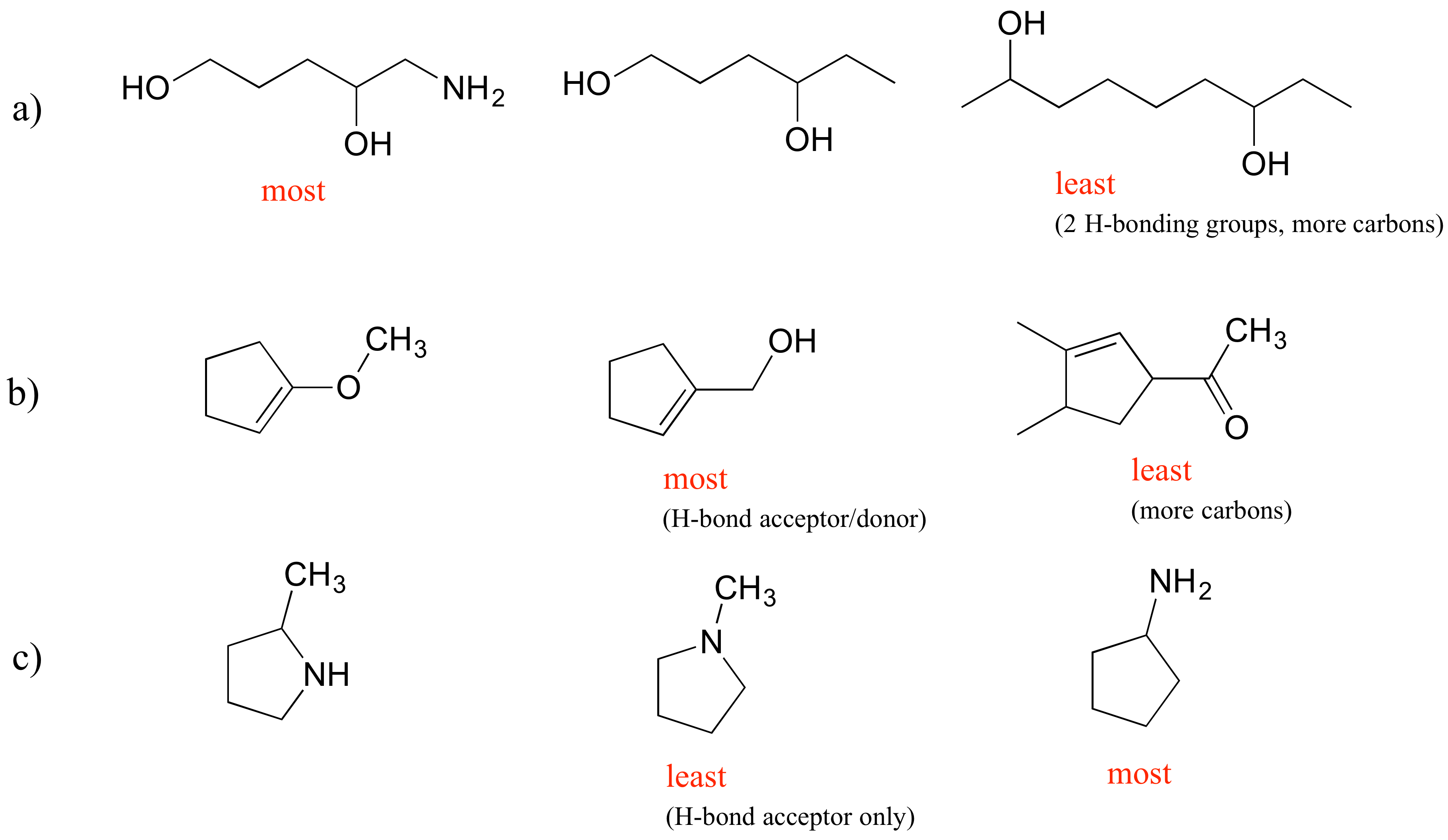
E2.30: The main factors in these examples are the number of H-bonding groups, whether groups are H-bond acceptors and donors or just acceptors, and the number of carbons.
E2.31: Ascorbic acid and niacin are water soluble (lots of hydrophilic groups relative to the number of carbons). Retinol is fat-soluble (only one hydrophilic alcohol group, a large hydrophobic hydrocarbon group.
E2.32: Aniline is basic and would be protonated (and thus cationic) in aqueous HCl. Charged species are generally water soluble. On the other hand, phenol is not basic and thus would remain as a neutral, water-insoluble molecule.
E2.33: Both alcohol solvents could form H-bonds with cyclohexanone, but isopropanol is less polar (it has three carbons), and thus would be the better solvent for the relatively nonpolar cyclohexanone.
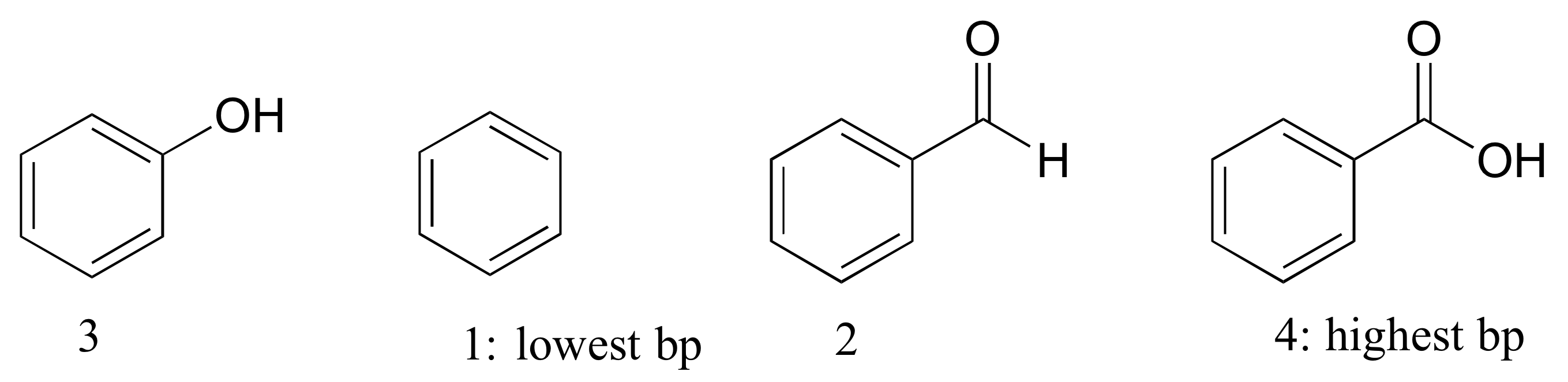
E2.34: Benzene has no polar groups and thus has the lowest boiling point (nonpolar interactions only holding molecules together). Benzaldehyde can form intermolecular dipole-dipole interactions; phenol and benzaldehyde can both form intermolecular H-bonds, but benzaldehyde has more dipole-dipole interactions due to the additional oxygen atom.