6.4A: Overview of Chemical Tests
- Page ID
- 95739
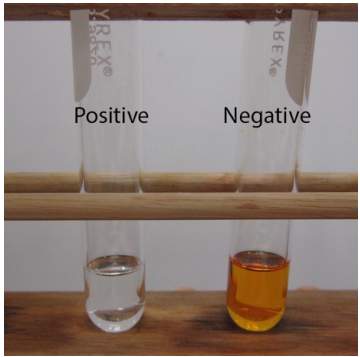
Before spectroscopic analysis (IR, NMR) became commonplace in the organic chemistry lab, chemical tests were heavily relied upon to support compound identification. A chemical test is typically a fast reaction performed in a test tube that gives a dramatic visual clue (a color change, precipitate, or gas formation) as evidence for a chemical reaction. For example, addition of an orange chromic acid reagent to some compounds causes the chromium reagent to change to a blue-green color (Figure 6.37a). This is considered a "positive" test result, and in this case indicates the presence of a functional group that can be oxidized (alcohol or aldehyde). A negative test result is retention of the original color of the reagent, in this case the orange color (Figure 6.37b).
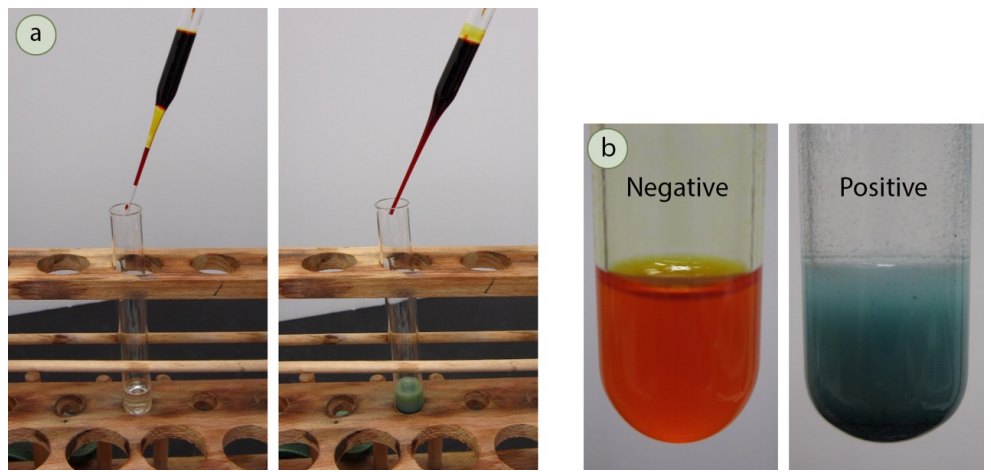
Performing chemical tests is commonly done in the teaching lab. Although the tests work well in general, when using a chemical test to support identification of a structure, caution should be used in interpretation of the results. For example, aldehydes are stated to give a positive result in the bromine test, which is when the compound turns the orange bromine solution clear. Figure 6.38 shows the reaction of two aldehydes with the bromine test: one gives a positive result (the left tube), and one gives a negative result (the right tube). Variation in chemical structure can sometimes interfere with "typical" results, leading to both false positives and false negatives. It is for this reason that spectroscopic methods are often more reliable in structure determination than chemical tests. Nonetheless, the ease of administration makes chemical tests preferable in certain applications, for example in roadside drug testing by police officers, and in environmental and chemical laboratories.