5.3C: Uses of Fractional Distillation
- Page ID
- 95720
Oil Refining
Crude oil (petroleum) is composed of mostly hydrocarbons (alkanes and aromatics), and is a tremendous mixture of compounds consisting of between 5 and 40 carbon atoms.\(^{11}\) The components in oil are incredibly useful as fuels and lubricants, but not when they are mixed together. Fractional distillation is used in oil refineries (Figure 5.41) to separate the complex mixture into fractions that contain similar boiling points and therefore similar molecular weights and properties. Gasoline, diesel fuel, kerosene, and jet fuel are some of the different fractions produced by an oil refinery.

Purification of Reagents and Products
Cyclopentadiene is used in many chemical reactions, including Diels-Alder reactions and polymerizations. The reagent is so reactive, however, that it undergoes a Diels-Alder reaction with itself in the reagent bottle to form dicyclopentadiene (Figure 5.42a). Therefore, chemical companies do not sell cyclopentadiene, and chemists are instead required to distill commercial dicyclopentadiene (Figure 5.42b) to reverse the dimerization reaction and obtain cyclopentadiene (Figure 5.42c).
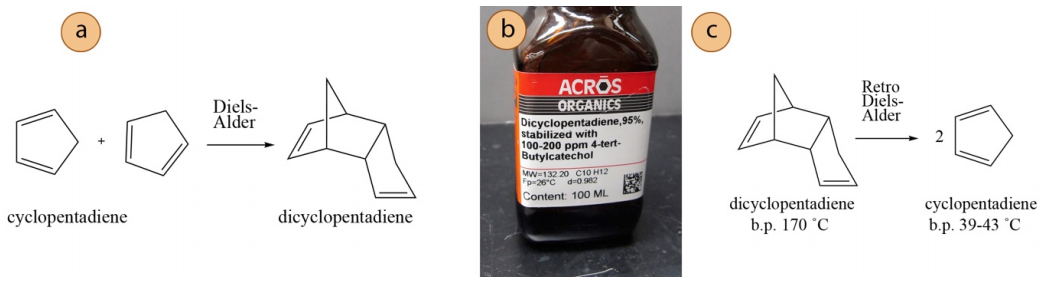
At temperatures above \(150^\text{o} \text{C}\) the dimer reverts to the monomer through a retro Diels-Alder reaction (driven by the favorable change in entropy, Figure 5.42c). Distillation can be used to remove the monomer as it forms. Although the two components (dimer and monomer) have dramatically different boiling points, the temperature required for the reverse reaction is too similar to the boiling point of the dicyclopentadiene that its vapor pressure cannot be ignored. Therefore, a fractional distillation is required for this process.
\(^{11}\)About \(6\%\) of crude oil contains hydrocarbons with greater than 40 carbon atoms, a fraction that eventually becomes used for asphalt.


