4.2: Overview of Extraction
- Page ID
- 93530
"Extraction" refers to transference of compound(s) from a solid or liquid into a different solvent or phase. When a tea bag is added to hot water, the compounds responsible for the flavor and color of tea are extracted from the grounds into the water (Figure 4.1a). Decaffeinated coffee is made by using solvents or supercritical carbon dioxide to extract the caffeine out of coffee beans. Bakers use the extract of vanilla, almond, orange, lemon, and peppermint in their dishes, essences that have been extracted from plant materials using alcohol (Figure 4.1b).

Figure 4.1: Examples of extraction: a) Tea, b) Baking extracts, c) Plant pigments extracted into water droplets after sprinklers hit a fallen leaf on the sidewalk.
In the chemistry lab, it is most common to use liquid-liquid extraction, a process that occurs in a separatory funnel (Figure 4.2). A solution containing dissolved components is placed in the funnel and an immiscible solvent is added, resulting in two layers that are shaken together. It is most common for one layer to be aqueous and the other an organic solvent. Components are "extracted" when they move from one layer to the other. The shape of the separatory funnel allows for efficient drainage and separation of the two layers.
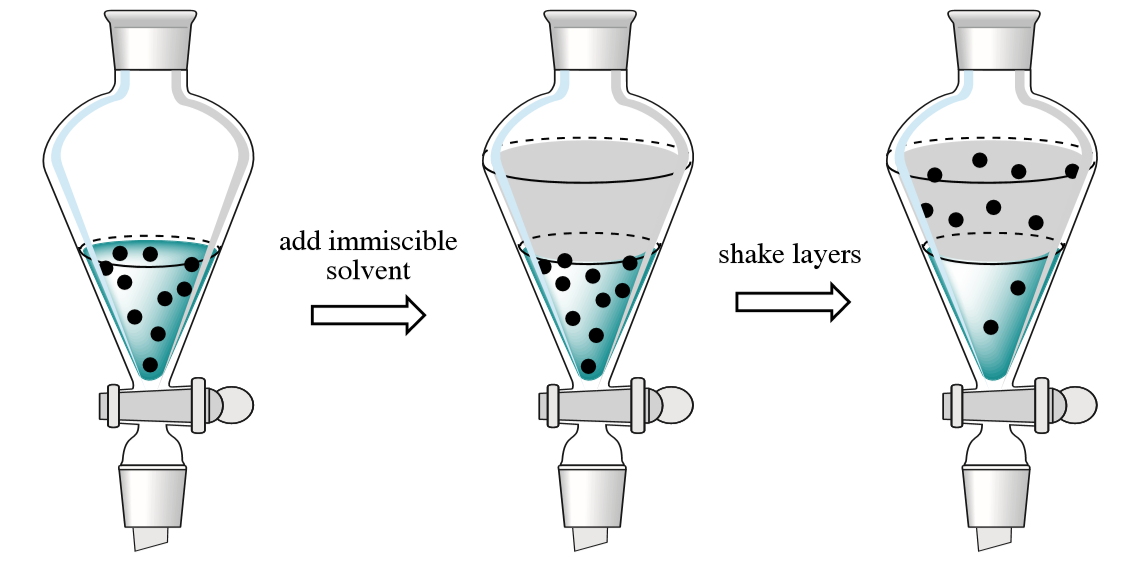
Compounds move from one liquid to another depending on their relative solubility in each liquid. A quick guide to solubility is the "like dissolves like" principle, meaning that nonpolar compounds should be readily extracted into nonpolar solvents (and vice versa). The compounds responsible for the taste and color of tea must be polar if they are readily extracted into hot water. When allowed to equilibrate between two liquids in a separatory funnel, the majority of a compound often ends up in the layer that it is more soluble.


