3.6C: Using Solvents Other Than Water
- Page ID
- 95768
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\)
This section describes a few key differences between a crystallization using water and one using volatile organic solvents. It is expected that readers have previously read or performed a crystallization using water as the solvent.
Ethanol, Methanol, Ethyl Acetate, and Hexanes
Ethanol, methanol, ethyl acetate, and hexanes are flammable and have moderate volatility, thus these solvents necessitate some different approaches than when using water as the crystallization solvent.

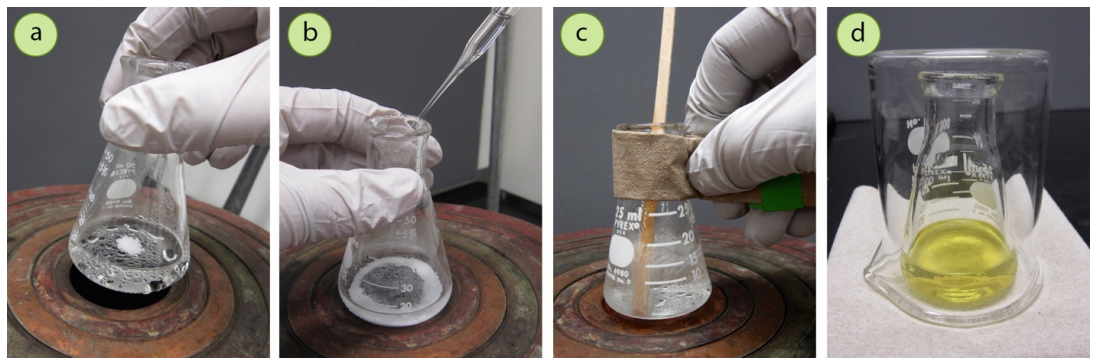
- Although a steam bath is the preferred heat source for these solvents, if a hotplate is chosen to be used carefully it's essential that you:
- Keep the hotplate on low (Figure 3.54a) and monitor the temperature of the solvent with a thermometer, being sure to patiently wait for the solvent to come to a boil instead of "cranking" up the heat.
- Use the hotplate in a fume hood to prevent a "blanket" of solvent vapors from forming around the hotplate, which have the potential to ignite.
- Monitor the hotplate the entire time while the solvent is heating.
- It may be more controllable to use a pipette to transfer portions of hot solvent to the solid instead of pouring (Figure 3.54b+c). Pouring has a greater possibility of spilling, and if solvent drips onto the hotplate surface, it has the potential to ignite. Since solvent tends to cool in a pipette, care must be taken to be sure the solution is returned to a boil before adding more solvent.
- All organic solvents have lower heat capacities than water, so tend to cool more quickly. Be sure to set the cooling sample atop several paper towels to encourage a slow crystallization (Figure 3.54d).
Diethyl Ether, Acetone, and Petroleum Ether (Low-Boiling)
Diethyl ether, acetone, and low-boiling petroleum ether are flammable and highly volatile, and thus necessitate further considerations than when using water or other organic solvents as the crystallization solvent.
- As these solvents have very low boiling points, they MUST be heated with a steam bath (Figure 3.55a), not a hotplate, or ignition may occur on the hotplate's surface.
- Steam is much hotter than is necessary to bring these solvents to a boil, and they must therefore be closely monitored during heating. To prevent too vigorous boiling, flasks may need to be lifted periodically off the steam bath, or simply hovered above the steam bath (Figure 3.55a) instead of resting directly atop the bath (Figure 3.55c). To control boiling, the steam rate should also be turned down.
- Using a Pasteur pipette with warm solvent is impractical with very volatile solvents, as liquid will undoubtedly drip out the end of the pipette before it is able to be delivered. Instead of heating the solvent beforehand, simply add cold solvent to the solid by pipette each time (Figure 3.55b), and then be sure to allow each portion to come to a boil before adding more.
Liquid may drip out the tip of a pipette even when dispensing cold solvent, which happens as solvent evaporates into the pipette's headspace, and the additional vapor causes the headspace pressure to exceed the atmospheric pressure. To prevent a pipette from dripping, withdraw and expunge solvent into the pipette several times. Once the headspace is saturated with solvent vapors, the pipette will no longer drip. - These solvents boil so readily that it's possible they will vaporize as quickly as a new portion of solvent is added (Figure 3.55c shows vigorous boiling). It may feel as if you keep adding solvent and are "getting nowhere" with the additions. It is important to be watchful of the solvent volume in the flask, and if the additions appear to be quickly "disappearing", raise the flask above the steam bath or turn down the steam to moderate the rate of heating.
- As these solvents are not much warmer than room temperature when boiling, they will cool very quickly. Thus, it may be helpful to cover the flask with an inverted beaker to create an insulating atmosphere in addition to setting the cooling flask atop several paper towels (Figure 3.55d).


