2.5C: Separation Theory
- Page ID
- 95705
General Theory
GC is an excellent analytical tool for separating mixtures in a sample. In this section are discussed the details of the separation, and expand upon the general discussion of Section 2.1B.
The GC column is what allows for the separation of a mixture's components by the instrument. It is composed of a thin, hollow tube approximately \(0.5 \: \text{mm}\) thick, and is lined with a very thin polymeric coating \(0.1\)-\(5 \: \mu \text{m}\) thick\(^{14}\) (Figure 2.80a). Columns can be 15-100 meters long\(^{14}\) (although commonly are 30 meters in length) and can be swapped in and out of an instrument depending on an experimenter's goals. There are columns specifically designed for analyzing poisons, biodiesel samples, or chiral compounds. An example of a typical academic capillary column is shown in Figure 2.80b, which is lined with a "dimethyl polysiloxane" polymer.
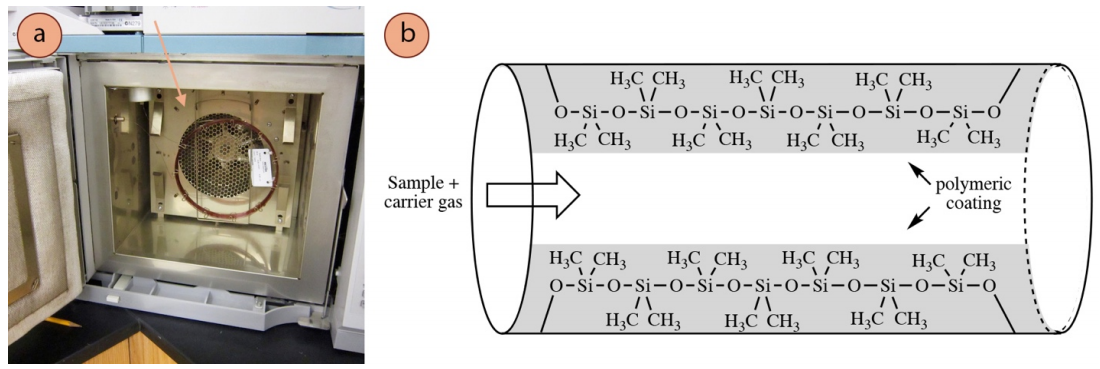
Like all forms of chromatography, compounds equilibrate between a stationary phase and mobile phase. In GC, the stationary phase is the polymeric inner coating of the column, and compounds can interact with this high boiling liquid through various types of intermolecular forces (IMF's). The mobile phase is the carrier gas (usually helium), which is continually pushed through the column. Samples enter the column in the gas phase, subsequently adhere to the column coating, and establish an equilibrium between the stationary an mobile phases (equation 4).
\[X_\text{(polymeric coating)} \rightleftharpoons X_\text{(gas)} \tag{4}\]
Compounds move into the mobile phase if thermal energy from the oven provides sufficient energy to overcome the intermolecular forces between the compound and the column coating. When in the mobile phase, compounds are swept with the flow of the carrier gas, and re-adhere to the column coating further down the column. Compounds can be thought of as bouncing from one position to the next until they exit the column.
As all compounds have the same length to travel before they exit the column, they all spend the same amount of time in the mobile phase. Thus, separation is due to the varying amount of time spent in the stationary phase, or how long compounds adhere to the column coating. Compounds that have weak IMF's with the column coating spend little time "hung up" in the stationary phase, as heat from the oven allows for their IMF's to be broken. They quickly emerge from the column and thus have a short retention time. Conversely compounds that can form strong IMF's with the column coating will favor the stationary phase, and take a long time to emerge from the column (they will have a long retention time).
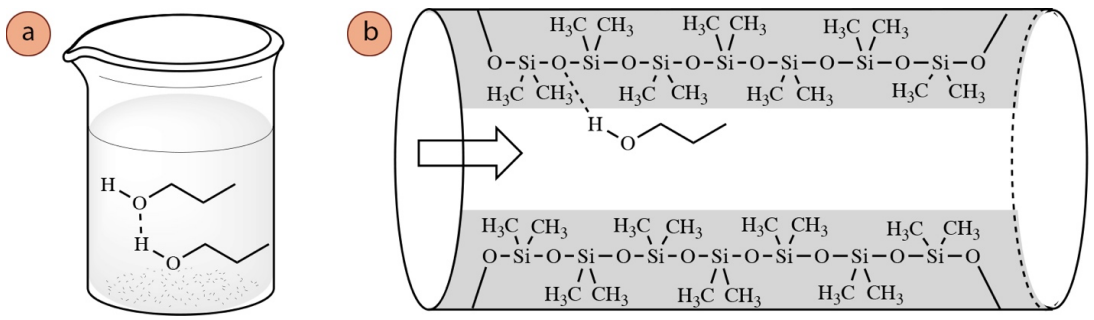
The type of IMF's experienced with the column coating often parallel the type of IMF's experienced in the pure liquid phase, meaning a compound that can hydrogen-bond in its liquid phase (Figure 2.81a) can also hydrogen bond with the column coating (Figure 2.81b). Therefore, the strength of interaction with the column coating correlates closely with a compound's boiling point, and boiling points can be used to predict the order of elution in GC.
In summary:
- Compounds that have weak IMF's with the column coating (low boiling points), spend little time in the stationary phase, exit the column early,and have shorter retention times.
- Compounds that have strong IMF's with the column coating (high boiling points), have longer retention times.
Structural Considerations
To demonstrate the effect of structural considerations, Figure 2.82 shows the GC spectrum of a mixture containing heptane, octane, nonane, and decane. The order of elution closely follows the trend in increasing boiling point. Heptane has the lowest boiling point of the series, and exits the column first (has the shortest retention time). As the chain length increases, so does the boiling point, and the retention times increase well (Table 2.9).
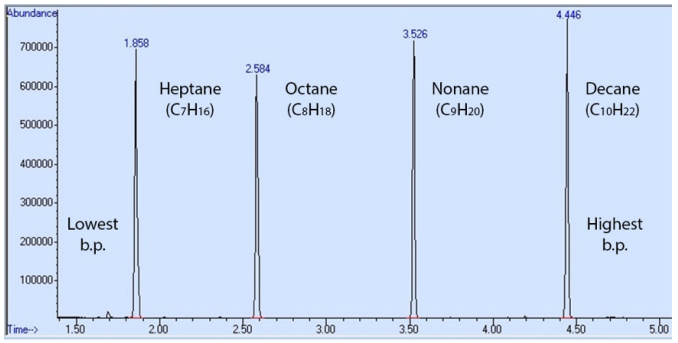
| Compound | Boiling Point (°C) | Retention Time (min) |
|---|---|---|
| Heptane (\(C_{7}H_{16}\)) | 98 | 1.858 |
| Octane (\(C_{8}H_{18}\)) | 125 | 2.584 |
| Nonane (\(C_{9}H_{20}\)) | 150 | 3.526 |
| Decane (\(C_{10}H_{22}\)) | 174 | 4.446 |
In another example, Figure 2.83 shows the GC spectrum of a mixture containing heptane, hexanal, and 1-hexanol. The order of elution again follows the trend in boiling point. 1-hexanol has the strongest IMF's, as it can interact with the column through both London dispersion forces (LDF's) and hydrogen bonds. This causes it to adhere to the stationary phase most strongly, resulting in the longest retention time. Heptane interacts with the column coating through weaker LDF's, so spends the least amount of time in the stationary phase and elutes first (Table 2.10).
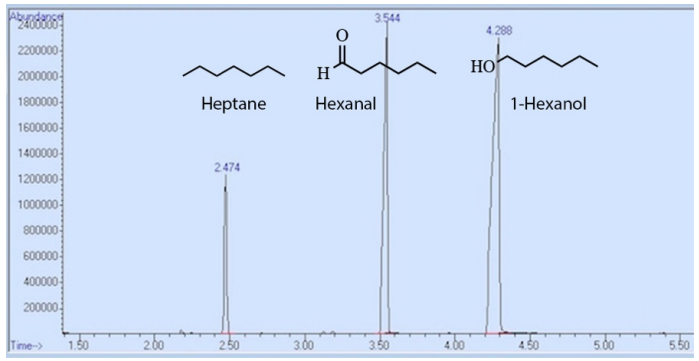
| Compound | Boiling Point (°C) | Retention Time (min) |
|---|---|---|
| Heptane | 98 | 2.474 |
| Hexanal | 130 | 3.544 |
| 1-hexanol | 155 | 4.288 |
\(^{14}\)D.C. Harris, Quantitative Chemical Analysis, 5\(^\text{th}\) edition, 1982, p 676.


