5.2D: Microscale Distillation
- Page ID
- 95714
There are a variety of methods used to distill small amounts of material \(\left( < 10 \: \text{mL} \right)\). The goal of all of these variations is to minimize the loss of material. A certain amount of sample is always necessary to fill the apparatus before distillation occurs, and this quantity (called the "holdup volume") normally condenses after cooling, often back into the original distilling flask. Therefore, minimizing the path length between distilling and receiving flasks can increase the recovery with distillation. Another approach to increase recovery on the microscale is to minimize the number of joints in the apparatus, which may not be perfectly airtight, and can contribute to leaking of material.
Most microscale versions are simple distillations, as use of a fractional column adsorbs too much material. Therefore, these techniques are generally used to remove non-volatile or very high-boiling or low-boiling impurities.
Semi-Microscale
A semi-microscale apparatus is essentially a macroscale apparatus but lacks the condenser (Figure 5.34). This shortens the path length between distilling and receiving flask. It can be used for \(5\)-\(10 \: \text{mL}\) of material.
The distillation needs to be done with care, as too high of temperatures may cause vapor to be lost out of the vacuum adapter.

Hickman Head
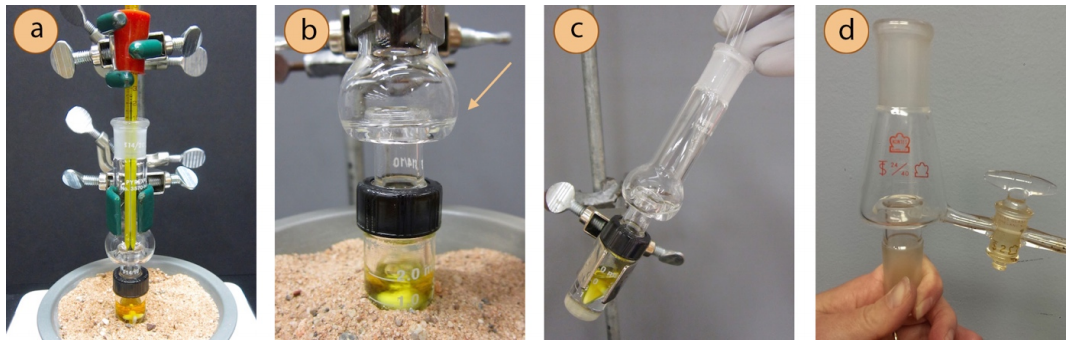
A Hickman head is provided in many microscale kits, and can be used to distill \(1\)-\(3 \: \text{mL}\) of material. It is often connected to a conical vial, using an O-ring and screw-capped connector to secure the joint (Figure 5.35a). The solution boils, condenses on the sides of the flask and collects in the lip of the Hickman head (indicated with an arrow in Figure 5.35b). The bulb of the thermometer should be positioned below the lip (Figure 5.35a), although it may be difficult to accurately measure the vapor temperature as so little material is used. After cooling, the flask must be tilted to retrieve the distillate by pipette (Figure 5.35c), and the lip holds at the most \(1 \: \text{mL}\) of distillate so may need to be emptied several times. Some Hickman heads have a side port so distillate can be removed without stopping the distillation (Figure 5.35d).
Short-Path Distillation
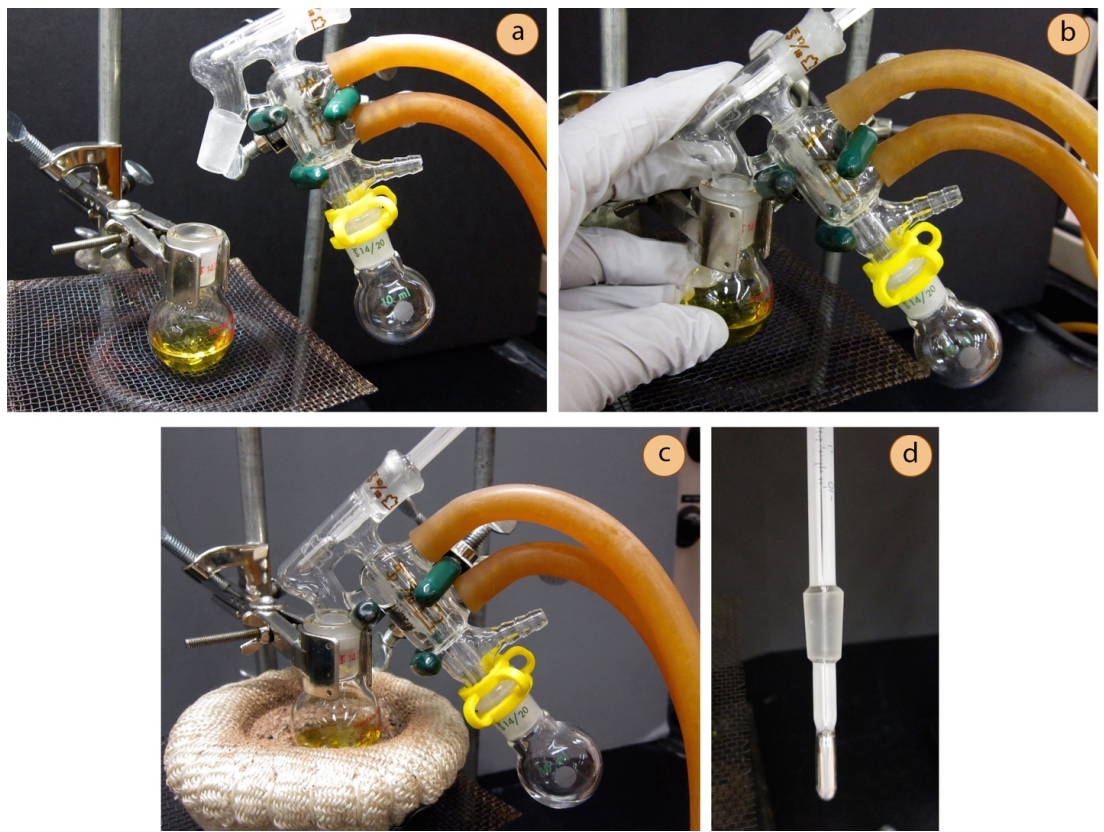
An all-in-one distillation apparatus, called a "short-path distilling head", is sometimes used to distill small quantities of material (Figure 5.36). These pieces of glassware are quite expensive ($200 each),\(^{10}\) and so are normally used in research settings, not teaching labs.
When assembling the glassware, it is important that the joint connecting the distilling flask to the apparatus is held securely: verify it is not loose by grasping the pieces with your fingers (Figure 5.36b). The apparatus requires a thermometer that has a ground-glass fitting (Figure 5.36d).
\(^{10}\)Prices were found in the Chemglass catalog in March 2016.


