3.5D: Cooling Slowly
- Page ID
- 95765
After a solution is dissolved in the minimum amount of hot solvent and filtered (if applicable), the solution should be cooled as slowly as possible (keeping in mind time limitations in a lab). To achieve this goal:
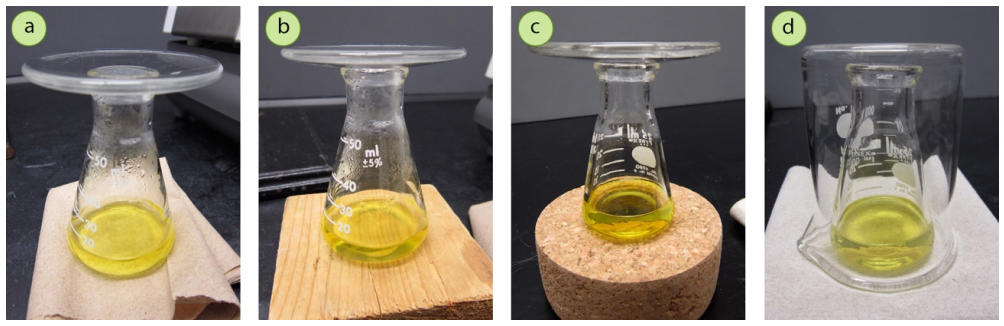
The mouth of the Erlenmeyer flask should be covered with a watch glass (Figure 3.43a). This retains heat just like placing a lid on a boiling pot. The watch glass also prevents excess evaporation of low-boiling solvents. Condensation can often be seen on the watch glass as the solution cools, which is evidence that warm vapors are trapped with this method.
The flask should be placed on a non-conductive surface so heat is not wicked away from the bottom of the flask. The flask can be placed atop a paper towel folded several times, a wood block, or on an inverted cork ring (Figure 3.43a-c). For solutions that cool very quickly (e.g., when using solvents with low boiling points like diethyl ether and acetone), the flask may also be covered by an inverted beaker (Figure 3.43d) to create an insulating atmosphere around the flask. This is not generally necessary for solvents with relatively high boiling points like water and ethanol.
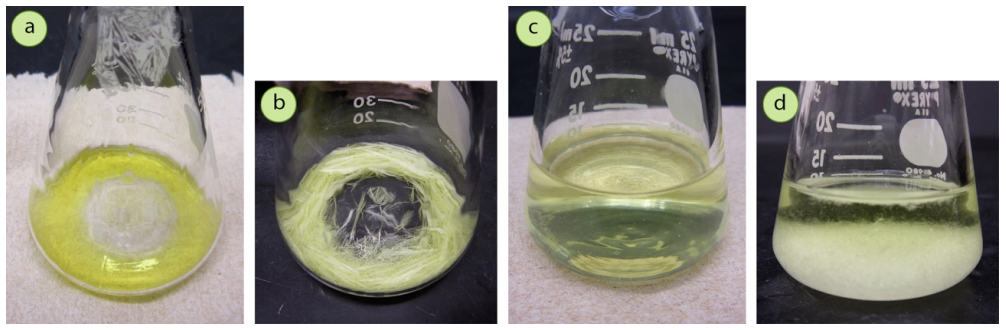
The correct flask size should be used so that the quantity of hot solvent used reaches a height of more than \(1 \: \text{cm}\) in the flask. If the solvent level is too shallow during crystallization, the high surface area will cause the solution to cool and evaporate too quickly (Figure 3.44b). It will also be difficult to filter a shallow volume. Ideally the solution should be to a height of at least \(2 \: \text{cm}\) in the flask, which allows for maintenance of heat by the solution's interior (Figure 3.44d). It is common to use between 10-50 times as much solvent as sample, and a rough guide is to use a flask where the sample just covers the bottom in a thin layer.