1.18: Glycosides, Disaccharides, Polysaccharides
- Page ID
- 81784
Glycosides
Last time we explored the structural characteristics of monosaccharides. We saw that the major stereochemical features of aldohexoses and aldopentoses are usefully described by Fischer projection formulas, but we learned that the structures of these compounds must also be understood as cyclic hemiacetals. Today we'll look in more detail at the chemistry of that hemiacetal linkage. In particular, we'll recall how hemiacetals are converted to acetals. We'll find that these acetal linkages are what holds di- and polysaccharides together.
Let's begin by remembering the reaction sequence which links aldehydes and alcohols, hemiacetals, and acetals.
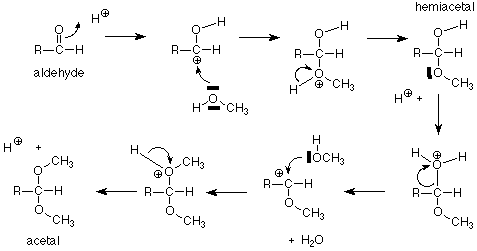
For our purposes, the key feature is the conversion of a hemiacetal and an alcohol to an acetal, with the concurrent release of a molecule of water. If we apply this feature of the scheme to a solution of glucose in methanol (with a trace of acid catalyst included), we get:
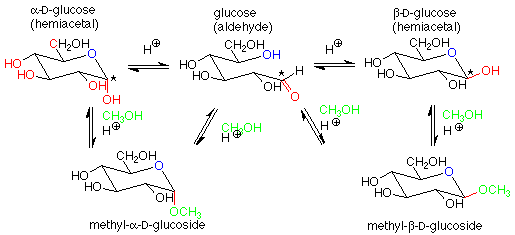
The acetal products are called "glycosides." If the sugar used is glucose, they are "glucosides." There are several reasonable mechanisms for these conversions and we will not look at them in detail. Keep in mind that the conversion between a hemiacetal and an acetal requires an acid catalyst. The conversion between an aldehyde and a hemiacetal is catalyzed either by base or by acid. Conditions can be arranged to produce either the alpha or beta stereochemistry in the glycoside.
Glycosides are very common in nature. Besides the di- and polysaccharides we will look at later, it is very common for glucose (or other sugars) and an alcohol to form an acetal linkage. Often this improves the water solubility of the alcohol and makes it easier to excrete. This is the case with cholesterol:
Reducing and Non-Reducing Sugars
There is another important difference between the hemiacetal and acetal linkages in sugars and saccharides, and that is their reaction with mild oxidizing agents. Aldehydes are fairly easy to oxidize to carboxylic acids, while acetals (which have no carbonyl group) are quite difficult to oxidize. The oxidizing agents used in carbohydrate chemistry are typically copper(II) compounds which are reduced to copper(I) oxide. Sugars which are oxidized by these reagents are called reducing sugars because they reduce the copper(II) to copper(I).
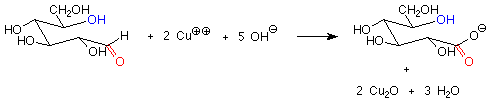
These reagents are used in basic solution, so that hemiacetals and aldehydes are in equilibrium. This means that the cyclic hemiacetal form of a sugar will produce an equilibrium amount of the open-chain aldehyde form, which will then reduce the copper(II) to copper (I) and give a positive test. A hemiacetal form is thus a reducing sugar.
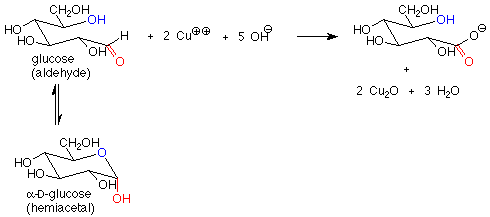
In contrast, acetal forms (glycosides) are not reducing sugars, since with base present, the acetal linkage is stable and is not converted to the aldehyde or hemiacetal. The outcome is that in a reducing sugar the anomeric carbon is in an aldehyde or hemiacetal. In a non-reducing sugar, the anomeric carbon is in an acetal.
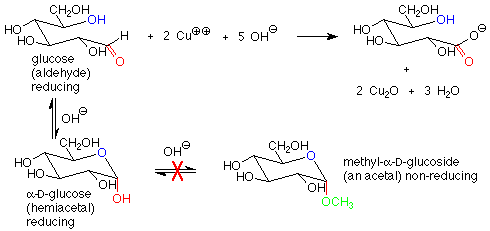
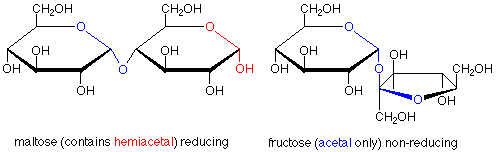
The characterization of sugars as reducing or non-reducing is gives useful clues as to their structures. Consider the disaccharides maltose and fructose. Maltose contains a hemiacetal functional group and is a reducing sugar. In fructose, both anomeric carbons are in acetal functional groups, so fructose is a non-reducing sugar.
Disaccharides
This brings us to the topic of disaccharides. The linkages between the monosaccharide ring units in disaccharides are acetal linkages. We can envision them as being made by the formation of an acetal from a hemiacetal and an alcohol. For this purpose, the hemiacetal includes the anomeric carbon of a monosaccharide and the alcohol role is played by a specific OH group of a second monosaccharide. The formation of maltose from two molecules of glucose is an example of this:
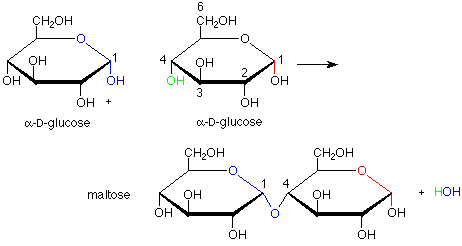
There are several intriguing features of this conversion. First, it is catalyzed by the enzyme maltase. The term "catalyzed" implies that enzyme speeds up the reaction in both directions, so that both formation and hydrolysis (conversion from acetal to hemiacetal using a molecule of water) are faster with the enzyme. Enzymatic catalysis is usually also very specific. In this case, that specificity shows up in the fact that the new acetal linkage has the alpha configuration, not the beta (and correspondingly, maltase catalyzes the hydrolysis of an alpha linkage but does nothing to the beta linkage). Also, only the OH group on the number four carbon atom is used as the alcohol when others, such as the ones on carbons 1, 2, 3 and 6 might have been used. This suggests that the enzyme holds the two molecules of glucose in specific positions so that only the OH on carbon 4 of one glucose can reach the anomeric carbon of the other glucose.
Fructose provides an example of a disaccharide in which the acetal linkage joins the anomeric carbons of a glucose molecule to the anomeric carbon of a fructose molecule. In this case there is no hemiacetal functional group, so fructose is a non-reducing sugar. Also, here one of the rings has five members rather than six, showing that the cyclization of fructose from the open-chain form to the hemiacetal cyclic form uses the OH at carbon 5 and the carbonyl carbon 2.
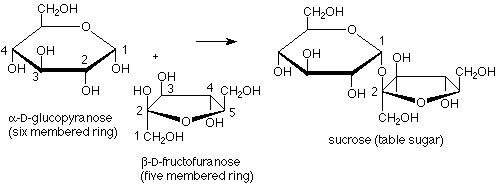
We can also look more carefully at fructose. In its cyclic form the anomeric (hemiacetal) carbon is involved in two carbon-carbon bonds. This means that when we open the molecule up to its open chain form the anomeric carbon becomes a keto carbonyl group. Fructose is thus an example of a ketose, a sugar in which the carbonyl group is a ketone rather than an aldehyde.

Polysaccharides
If we now return to our first look at polysaccharides, we can see that amylose starch is composed of many glucose monosaccharide units which are linked together by acetal functional groups involving the anomeric carbon of one glucose and the number four carbon of the next glucose. Repetition of this pattern many times gives the polymer.
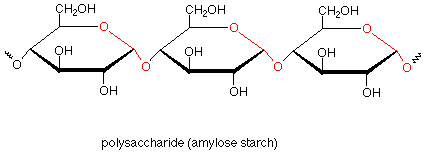
Amylose is a linear polymer with few branches. In amylopectin, another type of starch, there are branches which involve acetal linkages through the oxygen on carbon 6. Glycogen, sometimes called animal starch, is a similar polymer found in animals as a storage medium for glucose. Glycogen is even more highly branched than amylopectin.
Hydrolysis of starch involves the cleavage of the acetal functional groups with the addition of a molecule of water for each acetal linkage and the production of many molecules of glucose. This is done by the enzymes called glycosidases which are found in saliva. These enzymes work only on alpha acetal linkages and do not attack beta linkages. Such beta linkages are found in cellulose. Since our glycosidases are unable to hydrolyze the beta linkages in cellulose, we cannot digest cellulose, even though it is also a polymer of glucose.
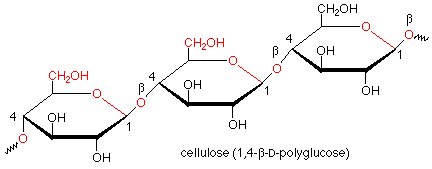
Of course, there are enzymes which hydrolyze the beta linkages in cellulose. Such enzymes are found in the bacteria which inhabit the stomachs of ruminants such as cattle and sheep, which makes cellulose digestible by ruminants. They are also found in fungi which rot wood.
The specificity of enzymes allows one monosaccharide, glucose, to be the building block for both starch, which we think of as a major source of energy in our foods, and cellulose, which we regard as a structural material in trees and a major component of paper. If we look at this in the context of the use of these materials in a plant, starch is found as a storage medium for glucose in seeds and tubers. It is used as a source of glucose both for energy and as a raw material for cellulose as the plant sprouts and enters its initial growth period. Enzymes specific for alpha linkages present in the sprouting plant hydrolyze the starch to glucose, as they do in the malting process used in beer and whisky production.
The cellulose produced as the plant grows is a major structural component of the plant. It must be quite stable if it is to serve that purpose, so enzymes specific for the alpha linkage do not attack its beta acetal functional groups and it is not readily hydrolyzed. The small stereochemical distinction between the alpha and beta linkages leads to very large consequences in the chemistry and function of starch and cellulose.


