1.1: Carbonyl Group - Notation, Structure, and Bonding
- Page ID
- 81767
Introduction
Organic chemistry is the chemistry of the element carbon. The compounds formed from carbon and a few other elements (O, N, P, S and H) form the chemical basis for living systems. Most therapeutic drugs are organic compounds. Organic polymers, whether obtained from nature or by synthetic means, are extremely important economic materials. These include plastics, rubber, glues, starch, cotton, and wood, as well as the proteins we must have in our diet.
Why is it called "organic" chemistry? Historically, when chemists discovered the Law of Definite Proportions at the beginning of the 19th century, it appeared that this law did not apply to the various compounds they had isolated from plant and animal sources. Carbon compounds can be so complex that the ratios of elements in them did not appear to be simple numbers. For example, ordinary table sugar has the molecular formula C12H22O11, not the kind of simple ratio seen with the oxides of copper, Cu2O or CuO, for example. Chemists imagined that organic compounds were held together by a mysterious "vital force".
The beginning of the end of the "vital force" hypothesis is generally considered to be Friedrich Wöhler's synthesis of urea in 1828. He started with lead cyanate, which is about as "dead" as any chemical can be, and ammonium hydroxide or chloride, also "dead", which generated ammonium cyanate, NH4+OCN- (empirical formula CH4N2O) (still "dead"). When he heated the ammonium cyanate, he got urea, H2NCONH2 (molecular formula also CH4N2O, but atoms arranged differently). Urea is just what the name sounds like, a major ingredient in urine, and was thought at the time to be a purely "organic" chemical. Other syntheses of "organic" compounds from "inorganic" materials soon convinced chemists that organic compounds obeyed the same laws of chemistry as other chemicals.
Although chemists gave up the "vital force" hypothesis at least 150 years ago, a shadow of it lingers on in the popular notion that "natural" organic materials are somehow safer or more healthful than synthetic chemicals. This popular notion ignores the fact that we would not know, for example, what vitamin C is if we could not find out its molecular structure, synthesize it, and show that the synthetic material is in every way identical with the vitamin C that the famous Hungarian chemist Szent-Gyorgy (pronounced "Saint-George") first isolated from Hungarian peppers. This bit of popular culture also ignores the toxicity of nicotine, strychnine, pufferfish toxin, and botulism toxin, the last of which is the most poisonous chemical known. You might even call the AIDS virus an "organic chemical", since it has a known chemical structure, though its ability to reproduce itself in the human body (and to undergo rapid molecular evolution to defeat immune response or chemical inhibitors) makes it far more sinister than a mere poison.
You will be learning and applying the principles which govern the structure of organic compound and relating your understanding of structure to the reactions--the changes in structure--which happen when specific portions of organic compounds interact with other chemical substances. We will spend the first several weeks of the semester looking at a group of organic compounds which share a common structural element--the carbonyl group.
Structural Principles
First, though, we need to review a few structural characteristics of the carbon atom. These are ideas which were part of your general chemistry courses, but it will help if we briefly restate them.
- Carbon is tetracovalent. That means that a carbon atom typically makes four bonds to other atoms and that these bonds are covalent--formed by sharing an electron pair between the two atoms joined by the bond. Such arrangements provide eight valence electrons for a carbon atom, so that it's electronic configuration is like that of the very stable noble gas neon. Similarly, hydrogen forms one covalent bond, oxygen two, and nitrogen three.
- Carbon can form multiple covalent bonds. That is, a single carbon atom can form a double (to C, O or N) or triple (to C or N) bond to another atom. A double bond would involve two electron pairs between the bonded atoms and a triple bond would involve three electron pairs.
- Bonds between carbon and atoms other than carbon or hydrogen are polar. That is, in a bond between carbon and oxygen or nitrogen the electrons are closer to the more electronegative element (oxygen or nitrogen) than to the carbon, so the carbon has a slightly positive charge. (Fluorine is the most electronegative element, and the elements close to fluorine in the periodic table are also quite electronegative.)
- Bonds between one carbon atom and another and between a carbon and a hydrogen are non polar. That is, the electron pair forming the bond is quite evenly shared by the atoms.
- We can predict the geometry of the bonds around an atom by using the idea that electron pairs and groups of electron pairs (such as in double or triple bonds) repel each other (Valence Shell Electron Pair Repulsion--VSEPR--Theory).
Representing Structures
Now, let's apply some of these ideas to a small organic compound, formaldehyde. The molecular formula (composition by element) of formaldehyde is CH2O. If we interpret this literally, reading from left to right, we get something like this (bonds are indicated by lines):
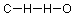
If we check this against our understanding of how many bonds each atom should form, we find that the carbon has one bond where we expect four, the hydrogens have two bonds instead of one, and the oxygen has only one instead of the expected two bonds.
A better approach is to draw four bonds to carbon and then think how the hydrogens and the oxygen might be linked to the carbon. If we connect the carbon and oxygen by a single bond, we get:
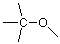
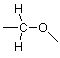
Here the lines which don't connect to more than one atom represent unused valences. We have four such unused valences and only two hydrogens to use them, so we'd be stuck with something like (there are other possibilities, but none based on this skeleton that work well):
If we remember the possibility that carbon and oxygen can make a double bond, we can check out a skeleton like:
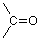
Now we have two unused valences and two hydrogens to connect to them, so by doing so we arrive at this structure for formaldehyde.
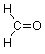
The structural unit made up of a carbon joined by a double bond to oxygen is known as a carbonyl group (often represented as "C=O"). We will spend the next several weeks on the chemistry of this group as it is found in somewhat different structural situations
We can learn from this process that converting a molecular formula to a structure is best done by working the atoms which can form more than one bond first, then checking each trial skeleton against the typical numbers of bonds for a particular atom. Those that pass that test can be further tested by balancing the number of unused valences against the remaining atoms so as to come out even.
More Carbons
Let's look at a more complex case, one with several carbon atoms. As you will learn from studying the section on isomerism in Brown (Section 3.2), a molecular formula is not enough to specify the structure of an organic compound which includes more than three carbons. We need information about which atoms, particularly carbons, nitrogens and oxygens, are connected to each other. This is often represented in a condensed formula like:
To take a more detailed look at the structure of this compound, we need to expand its representation. (See Brown, Section 3.4) As we have learned to do, we begin by ignoring the hydrogens and focusing on the carbons and the oxygen to arrive at a skeleton. A trial skeleton might be (where the unused valences are again shown as lines that connect to only one atom):
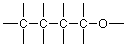
If we compare this skeleton to the condensed formula above, the left end carbon seems to be associated with three hydrogens in the condensed formula and there are three unused valences on the left end carbon as shown in the skeleton, so that matches well. Similarly, the two middle carbons have two hydrogens each, and there are two unused valences on each of those carbons in the skeleton. If we match all these up we arrive at:
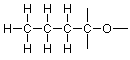
The right end carbon and the oxygen are troublesome, though. We have only one hydrogen left and there are three unused valences to deal with. We've seen this situation before when we were working on formaldehyde and we can use the same idea here, so that we try a double bond between carbon and oxygen. That gives us this skeleton:
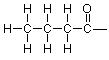
Adding the final hydrogen, we arrive at this expanded structure:
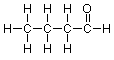
Bond-Line Structures
It is often inconvenient to show all the carbons and hydrogens in detail, especially since we will learn soon that the parts of a molecule which are made up of only carbon and hydrogens joined by single bonds do not play a significant role in the reactions of that molecule. (These portions of the molecule are known as "R-groups.") We can represent these atoms and how they are connected by using an abbreviated structural type known as a bond-line structure. Such a structure for the compound we just worked with is shown below:
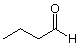
In a bond-line structure, carbons are shown by the "empty" ends of lines and by junctions (corners) between lines. Letters at the end of a line represent atoms of the designated heteroatom (Heteroatoms are atoms other than carbon or hydrogen. Hydrogen is often shown where necessary for clarity.) Double or triple bonds are represented by two or three parallel lines joining the same two atoms. Hydrogens are added as needed to fill up the remaining unused valences.
We can "flesh out" the skeleton above by first filling in the carbons at the corners and "empty" ends:
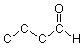
Then we count how many bond each carbon has showing, and add enough hydrogens to each carbon to bring its number of bonds up to four. (For other atoms like oxygen or nitrogen which can make more than one bond, hydrogens are added as needed to arrive at an appropriate number of bonds.) For example, the left end carbon has one bond showing, so we need to add three hydrogens:
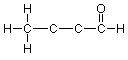
Continuing in the same way with the middle carbons, each of which has two bonds showing, we arrive at the final expanded structure:
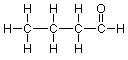
Notice that we didn't need to do anything to the right end carbon (the "carbonyl" carbon) or the oxygen, since these atoms already have the appropriate number of bonds showing.
Sometimes we'll represent a molecule by showing its R-groups in a condensed fashion and its reactive parts (functional groups, see next lecture) in an expanded fashion:

All these representations are useful, but we will commonly use the stick or line representation because it is economical to draw. You should practice converting between stick or line structures, condensed structures, expanded structure and the structures which show R-groups in condensed way and functional groups expanded. Try it with these examples:



