2.5: SUBLIMATION
- Page ID
- 135946
Sublimation is a purification technique for solids and in the context of this book, for organic compounds with lower melting points. Sublimation describes the process of a solid becoming a gas, without passing through the liquid state. The gas phase is then typically crystallized on a cold surface. However, many sublimations require reduced pressure, and we will primarily focus on that variation of sublimations. The reason for many sublimations happening under reduced pressure, is because the sublimation point is decreased with decreasing pressure. To allow organic compounds to sublimate, and not simply melt, a reduced pressure is often necessary.
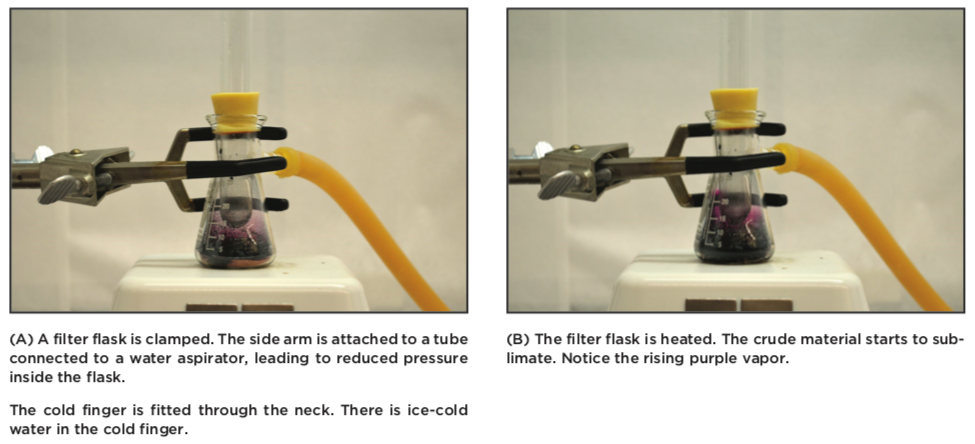
A crude, but efficient sublimation apparatus can be made from of a filter flask, where the side neck is connected to an aspirator or vacuum trap, and the neck is equipped with a cold finger (a tube with ice water, or other cooling material). The impure solids are placed in the bottom of the filter flask, and the cold finger is inserted. While an aspirator or pump is reducing the pressure inside the filter flask, the material is carefully heated on a hot plate (take care to avoid melting or boiling the material). The organic compound will start to sublime forming a gas. Once this gas reaches the cold finger, it will immediately crystallize on the cold finger, where it can be collected.

Troubleshooting sublimation
- The crude material does not sublime.
The most common reasons are insufficient heat, and/or the pressure is not low enough. - The sample sublimes, but the material crystallizes on the sides of the filter flask, and not on the cold finger.
The bottom of the flask might be hot enough, but the sides are not. The material is allowed to sublimate, but cannot reach the cold trap and is able to crystallize earlier. Insulation of the lower part of the filter flask can be appropriate.
- I don’t know where the sample went!
The crude material was added, but during the process of heating the flask, the material is gone, and there are no crystals. There can be several reasons, but the most likely contender is that the cold finger did not efficiently work as a trap. Lowering the position of the cold finger is a good solution.
- The crude material is boiling!
The system can be too hot, and less energy should be supplied to heat the system. Potentially the pressure should be reduced. - The product crystallizes on the cold finger, but looks pasty and wet and not crystalline.
The ice cold water in the cold finger has been in the finger for too long. The cold surface has started condensing water from the atmosphere, and once the product starts to reach the cold surface it is met by water. In general, the ice cold water should be added to the cold finger right before the sublimation starts to eliminate this problem.


