2.3: LIQUID-LIQUID EXTRACTION
- Page ID
- 135944
Liquid-liquid extraction involves the exchange of certain com- pounds between two solvents that are immiscible or only partially miscible. Liquid-liquid extraction is also very 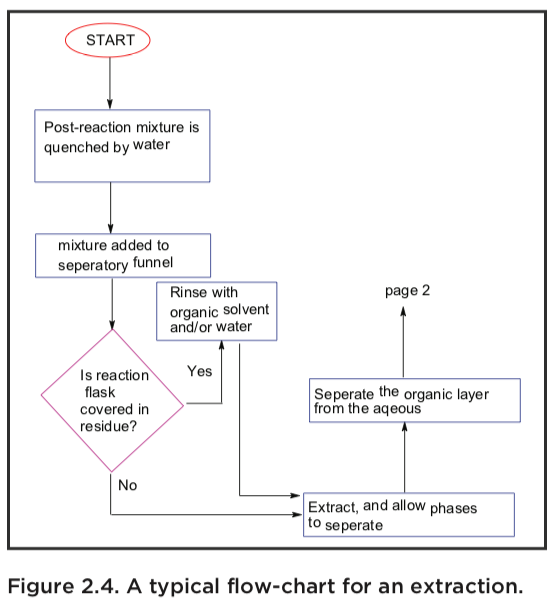 commonly used for washing an organic phase, for example to remove inorganic compounds, or to protonate or deprotonate bases or acids, respectively, so they become soluble in the aqueous phase. A very typical extraction flow diagram is shown below, where a reaction mixture is quenched with water, extracted (several times), washed with brine, dried, filtered and finally evaporated to yield a crude product or a pure product.
commonly used for washing an organic phase, for example to remove inorganic compounds, or to protonate or deprotonate bases or acids, respectively, so they become soluble in the aqueous phase. A very typical extraction flow diagram is shown below, where a reaction mixture is quenched with water, extracted (several times), washed with brine, dried, filtered and finally evaporated to yield a crude product or a pure product.
Liquid-liquid extraction (we will refer to it simply as extraction from now on) is typically conducted with one aqueous phase (either pure water, or an aqueous solution) and one organic phase. It is important to note that the desired compound (usually an organic molecule) can in theory be in either phase. It depends on both the nature of the compound, but also the nature of the aqueous phase. We will first examine some typical scenarios involving extraction, then move on to discuss technical details to keep in mind.
- Extraction of neutral compounds. If the desired organic compound is neutral (i.e. is neither acidic nor basic), the extraction sequence usually involves simply extracting with an organic solvent several times.
- Extraction of acidic compounds. If the desired compound is acidic, we can selectively deprotonate that compound by using an aqueous base. This will pull the deprotonated compound into the aqueous phase. Because very few organic compounds are soluble in water, we can discard the organic phase which now contains any byprod- ucts and/or unreacted starting materials. Acidification of the aque- ous phase can precipitate the desired product.
- Extraction of basic compounds. If the product is basic, we can perform a sequence very similar to the acidic compound above. We can protonate the basic compound by using an aqueous acid, pulling the protonated compound into the aqueous phase and discarding the organic phase. Neutralizing the acidic phase will depro- tonate the basic compound, which may precipitate, or may require a second extraction with an organic solvent.
The typical apparatus used in an extraction, is the separatory funnel. These come in many different sizes, but the typical size is 100 mL, which you will encounter in several labs at PSU. The funnel is equipped with a top and a nozzle, and the nozzle can be closed or opened with a stopcock. The funnel is narrow at the nozzle, which is a structural feature that allows us to accurately separate the two phases.

How to perform an extraction. For simplicity’s sake, we will assume that the product is a neutral compound.
- Make sure the stopcock is closed. Add a small amount of water or the aqueous solution you are using. Some- times the stopcock is improperly sealed, and you will find that it leaks. You would rather know that now, rather than after the organic material has been added.
- Add the reaction mixture, as well as water if necessary, and the required organic solvent. Make sure that the solutions added do not touch the inside of the top of the funnel, as solids can crystallize here and prevent the stopper to seal properly, leading to leaks.
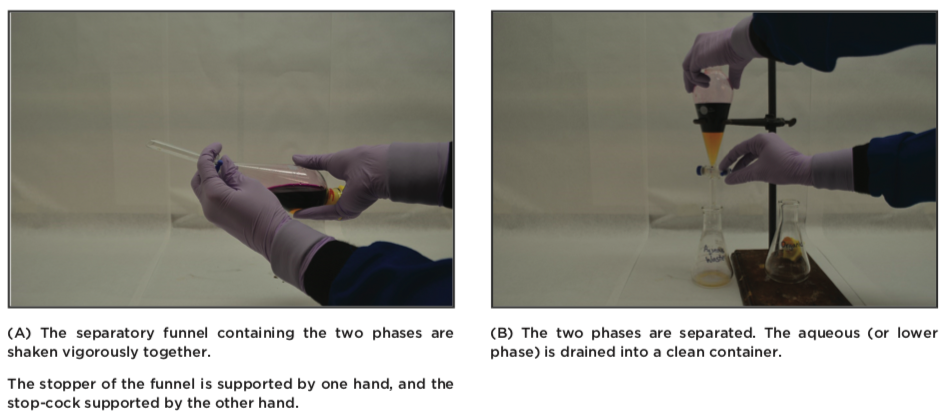
- The funnel is closed with a stopper, turned on its head, and shaken very carefully. The stopper is always supported by your hand.The pressure is released after each round of shake by opening the stopcock (point it upwards into the hood, but never towards yourself or someone else), and then closed again. Now the two layers are shaken vigorously. It is imperative that the two phases are mixed, because no compounds will exchange without a good mixing.
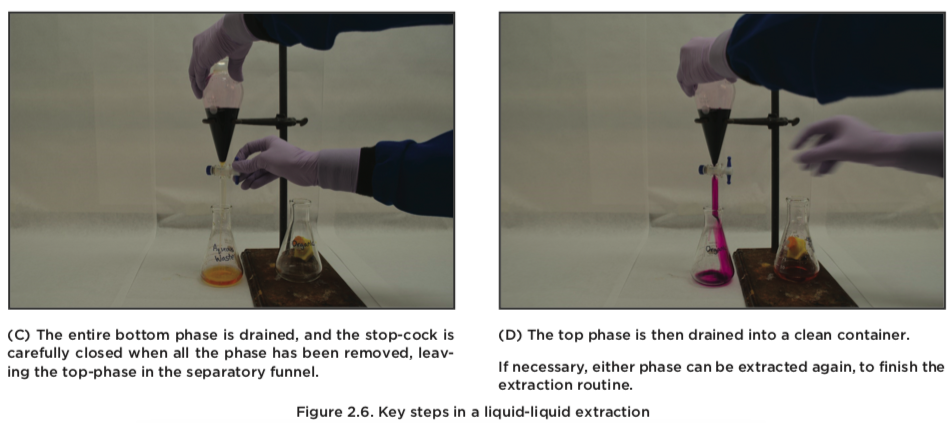
- The funnel is rested in a ring stand, and the stopper removed. The two phases are allowed to separate. The phases are removed by draining them out, one at a time.
- The aqueous phase can be extracted again, up to three times. The combined organic fractions are typically dried with a drying agent,9 then filtered, and the solvent evaporated.
9 A classic problem is that a student either has very little product after extraction ( because of improper mixing of phases) or that the student requires a vast amount of drying agent (not allowing the two phases to separate properly).
One common problem with extractions is one that does not arise from improper technique, but from the annoying behavior of certain compounds: they form emulsions. These usually appear between the two lay- ers, and are chemically composed of the two solvents, and other compounds found in the original solution. The emulsion can resolve itself over a few minutes, or be very persistent. In the latter case, it can sometimes be a good strategy to drain the emulsion out in the phase that does not contain the product, and then re-extract it several times
Let us say that we are extracting an aqueous solution with the organic solvent diethyl ether. Let us also say that the desired compound is organic, and in this example will be dissolved in the organic phase. A very persistent emulsion is formed. If we drain the aqueous phase and as little of the organic phase as we can (while still draining the emulsion out), we can obtain some organic phase without emulsion that is set aside. We then add the phase back to the separatory funnel and re-extract the aqueous phase that still contains some organic phase, as well as emulsion again. This usually disperse the emulsion.


