1.4: THE NOTEBOOK – MAKING SURE THE NUMBERS ADD UP
- Page ID
- 135938
At PSU, it is required to use and keep a notebook. This notebook will be used both in the preparation of the experiment, and during the experiment itself. We will focus on how to use the notebook in the prepara- tion phase of an experiment. In chapter 4.4 we will discuss the use of the notebook during the experiment.
When stepping into the lab, your notebook should contain the following:
- A title and a balanced reaction scheme, if a synthesis is planned. If not, the relevant chemical structures of the compounds of interest should be shown.
- A flow-diagram that outlines the experiment, where you have included the most relevant information.
- A table or overview that shows all chemicals handled, and their risk and hazard statements.
- A synthesis table that shows the quantities of the reagents/reactants that you plan to use, with room to fill in the quantities you actually used.
- A table that shows the properties of the reagents/reactants and reaction solvents used in the procedure.
The synthesis table should contain the most important physical properties of the reactant(s), reagent(s) and product(s). This table should contain the quantities you should measure, 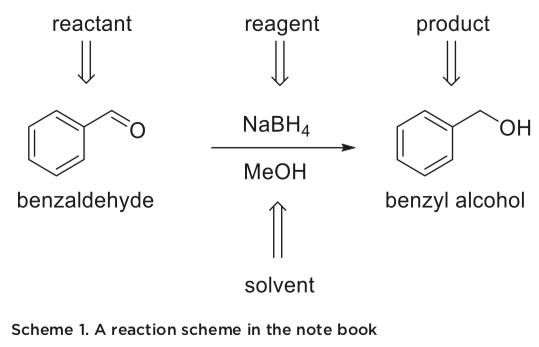 with room to add your actual measurements. This format allows you to order all the necessary measurements in the same place. An example is shown in table 2. There are many exam- ples where this information is important in lab. If you for example measure 0.4 mL of benzaldehyde, instead of the 0.3 mL the procedure called for, you need to scale the procedure up. That means that the amount of NaBH4 also must be changed. For this process, you need the density of benzaldehyde, the molecular weight of benzaldehyde to find the moles, and the molecular weight of NaBH4.
with room to add your actual measurements. This format allows you to order all the necessary measurements in the same place. An example is shown in table 2. There are many exam- ples where this information is important in lab. If you for example measure 0.4 mL of benzaldehyde, instead of the 0.3 mL the procedure called for, you need to scale the procedure up. That means that the amount of NaBH4 also must be changed. For this process, you need the density of benzaldehyde, the molecular weight of benzaldehyde to find the moles, and the molecular weight of NaBH4.
Table 2. Example of a synthesis table for reagent(s), reactant(s) and product(s)
| Compound | Mw [g/mol] | d [g/mL] | V [mL] | m [g] | moles [mmol] | Y ield [%] |
|
Benzaldehyde NaBH4 Benzyl alcohol |
106.7 37.8 108.1 |
1.044 - 1.044 |
0.30 _____ - - |
0.31 _____ 0.15 _____ 0.21* _____ |
2.9 _____ 3.9 _____ 1.9*_____ |
- - 65.6*_____ |
* The values for benzyl alcohol is based on the procedure, that states that a yield of 65.6% is expected.
The table for physical properties is used not only for the reagent(s), reactant(s) and product(s), but also reaction solvent, work-up chemicals and others. This table is very handy in the lab, as the physical properties of the chemicals play a large part in the work-up of any reaction.
Table 3. Example of a table for physical data
| Compound | State | Boiling point [°C] | d [g/mL] | Solubility in water [g/100 mL] | Solubility in diethyl ether |
|
Benzaldehyde NaBH4 Benzyl alcohol Diethyl ether Methanol |
Liquid Solid Liquid Liquid Liquid |
178 - 205 36 65 |
1.044 Not relevant 1.044 0.71 0.79 |
0.3 Decomposes 3.50 6.05 Soluble |
Soluble Non soluble Soluble - Soluble |


