9.3: Co- and Terpolymerization of Alkenes with Carbon Monoxide
- Page ID
- 168824
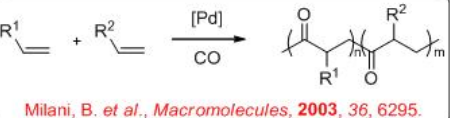
The catalytic copolymerization of alkenes with carbon monoxide to afford polyketones is of the industrial interest (Scheme \(\PageIndex{1}\)). Polyketones represent low-cost thermoplastics whose synthesis, properties and applications are thus the object of intense fundamental and applied research. The properties of polyketones can be modified by changing the nature or number of monomers, which makes them superior to polyalkenes, polyamides and polyacetals.
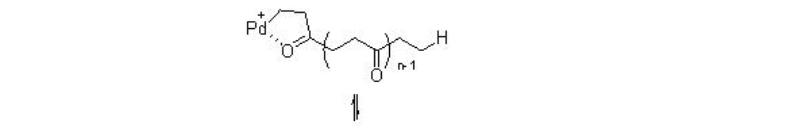
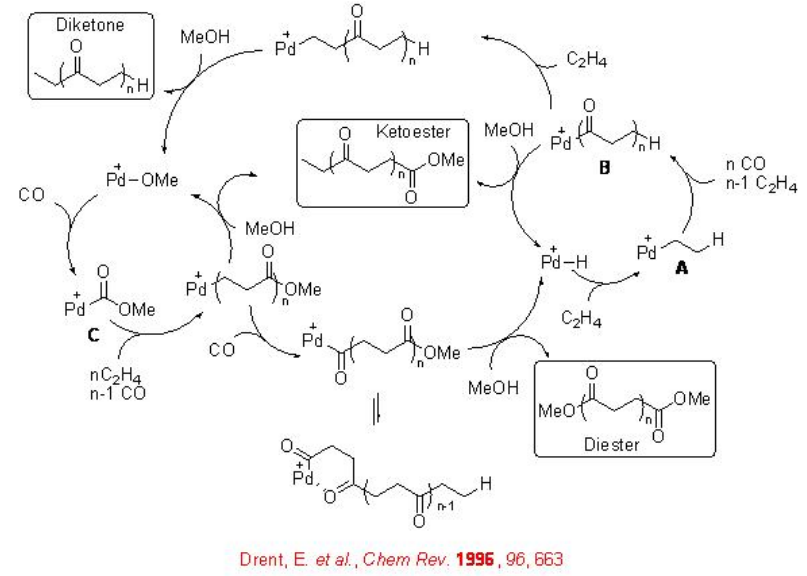
The reaction involves two competing catalytic cycles (Scheme \(\PageIndex{2}\)). One of the cycles initiates via a Pd-H species, in which, rapid insertion with ethylene with Pd-H leads to the formation of Pd-alkyl species A that reacts with CO to give Pd-acyl complex B . The latter irreversibly can insert with second ethylene molecule. Thus, the chain propagation occurs through alternating ethylene and CO insertions. Depending on the termination path, the catalytic cycle gives diketones or ketoesters. For example, methanolysis can lead to the formation of ketoester, while protonolysis can give diketones. While the second catalytic cycle initiates via a Pd-OMe species, in which, CO reacts with the Pd-OMe species to a form a Pd-carbomethoxy complex C . By this cycle ketoester is also produced along with copolymer diester that is produced via methanolysis of a Pd-acyl complex. Thus, the methonolysis is the main terminating step of the reaction and the ethylene insertion is the rate determining step of the reaction.
With respect to the CO/vinylarene copolymerization, the main features of the catalytic cycle are comparable to that of the CO/ethylene copolymerization. In particular, the chain propagation step is similar, although, the termination and initiation steps will depend on the nature of the alkenes.
9.3.1 Asymmetric Copolymerization of CO with Aliphatic Alkenes
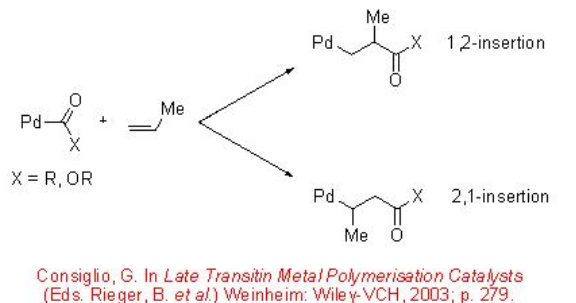
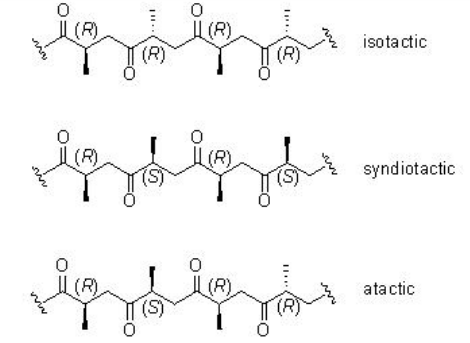
Unlike the reaction with ethylene, the CO/propene copolymerization can afford stereoregular copolymers. The mode of insertion of the propene into the Pd-acyl or Pd-carbomethoxy bond in a 1,2 or 2,1-fashion governs the regiochemistry (Scheme \(\PageIndex{3}\)). The stereochemistry of the reaction can lead to the formation of isotactic, syndiotactic or atactic structure (Scheme \(\PageIndex{4}\)).
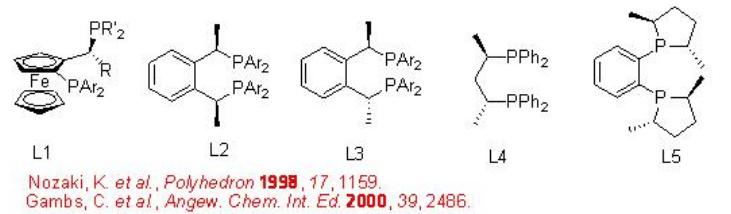
The best results are obtained utilizing catalyst containing bidentate phosphine ligands. The steric and electronic properties of the ligands control the activity of the catalyst and selectivity of the products. Scheme \(\PageIndex{5}\) summarizes some of the active ligands for the CO/propene copolymerization process that provide highly regio- and stereoselective co-polymers.
These catalysts are also effective for the copolymerization of CO with higher aliphatic 1-alkenes but slightly lower activity is observed compared to that with the reaction of propene. However, the regio- and stereoselectivities are found to be similar to that of propene reaction.
9.3.2 Asymmetric Copolymerization of CO with Vinylarenes
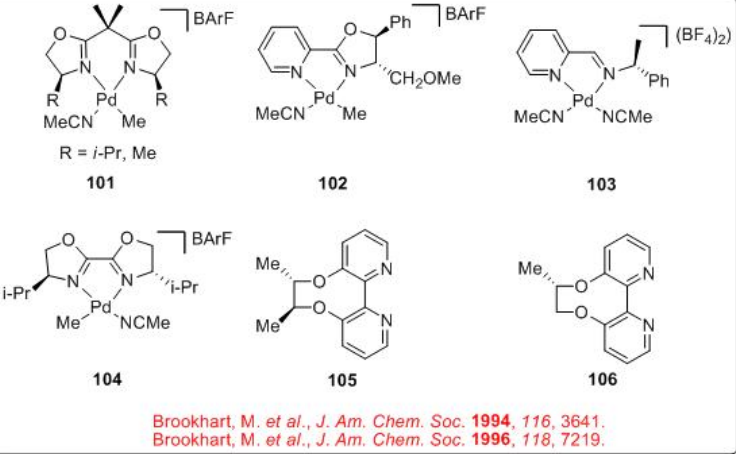
Unlike the CO/propene polymerization process that employs phosphine based ligands, the copolymerization of styrene with CO is generally found to be successful with dinitrogen ligands. Scheme 6 summarizes some of the successful dinitrogen based chiral ligands and chiral Pd-complexes for the CO/styrene copolymerization process.
9.3.3 Asymmetric CO/Alkenes Terpolymerization
The CO/alkene copolymer is packed in orderly that makes highly crystalline and very fragile. One of the ways to somewhat disturb the orderly crystal packing in the copolymer is the introduction two different kinds of alkenes so that they can have two types of units: CO/alkene1 and CO alkene2(Scheme \(\PageIndex{7}\)). Both chiral phosphines and chiral diamine ligands are found to be effective for these reactions.