9.1: Hydroformylation Reaction
- Page ID
- 168822
Carbonylation of the unsaturated substrates using transition metal catalysis provides powerful tool to produce fine chemical intermediates. The asymmetric carbonylation is among the most challenging homogeneous process and their potential is yet to be made. The Rh-catalyzed hydroformylation of alkenes together with the Pd-catalyzed hydroxy and alkoxycarbonylation of alkenes are the most famous examples for the asymmetric carbonylation reactions. The important difference between these reactions is the Rh-catalyzed hydroformylation is of greater industrial interest than the palladium based carbonylation process.
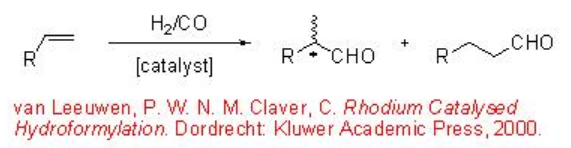
The conversion of alkenes to aldehydes is the largest volume homogeneous transition metal-catalyzed reaction. This process has been extensively explored and a number of methods and catalysts have been developed to control the regioselectivity in internal and terminal aldehydes (Scheme \(\PageIndex{1}\)).
9.1.1 Reaction of Vinyl Arenes
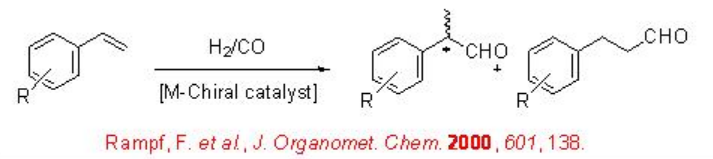
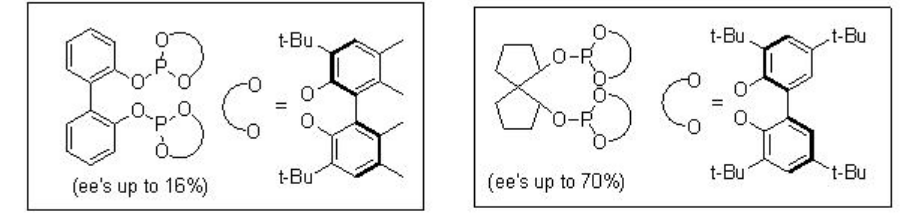
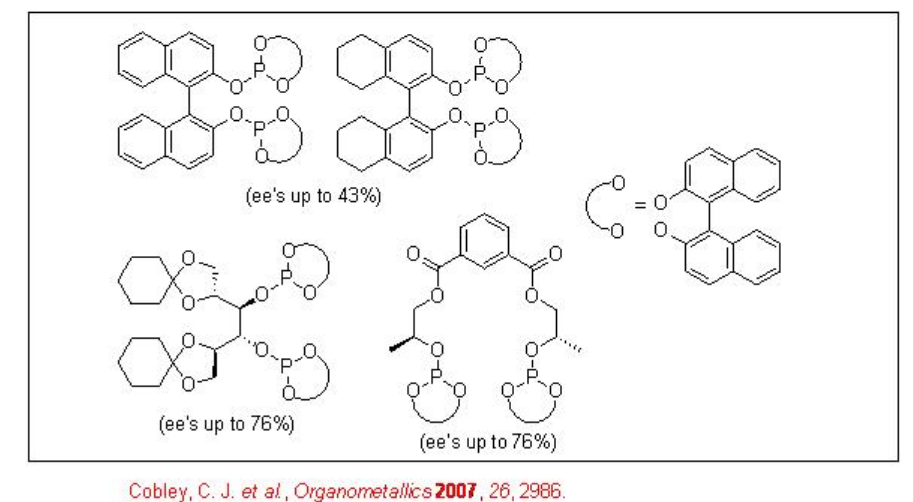
The asymmetric hydroformylation of vinyl arenes is an attractive route to afford optically active aldehydes, which are substrate precursors for the synthesis of high-value pharmaceuticals, agrochemicals, biodegradeful polymers and liquid crystals (Scheme \(\PageIndex{2}\)). Since the beginning of 1970, chiral Rh-diphosphine complexes have been used as catalysts for this transformation with moderate enantioselectivity (below 60%). From beginning of 1990, the use of bisphophacyclic ligands, diphosphites and phosphine-phosphite, has emerged as alternative for this reaction. Scheme \(\PageIndex{3}\) summarizes some of the new diphosphite ligands developed with biaryl, spiro, pyranoside, mannitol and macrocyclic backbones for the asymmetric hydroformylation of vinyl arenes with low to moderate success (ee's from 16% to 76%).
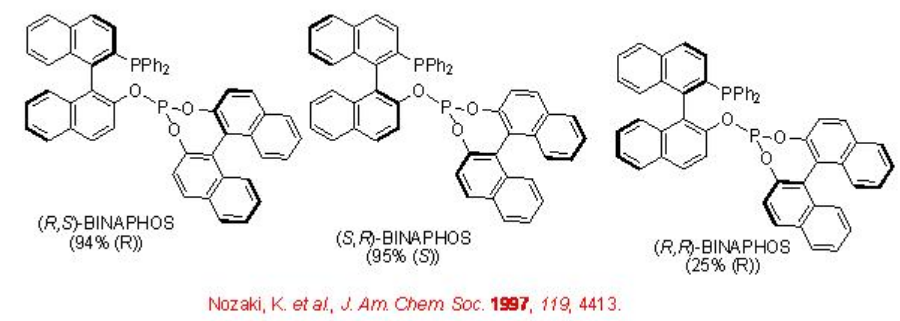
Scheme \(\PageIndex{4}\) summarizes some of successful phosphine-phosphite ligands for the asymmetric hydroformylation of vinyl arene. The enantioselectivity depends on the configuration of both the binaphthyl moieties. The best enantioselectivity is observed when the configurations of the two binaphthyl moieties are opposite.
9.1.2 Reaction of Vinyl Acetate
The reaction of vinyl acetate is more challenging compared to that of vinylarenes. This process affords 2- and 3- acetoxy propanals with high selectivity (Scheme \(\PageIndex{5}\)). Ethyl acetate and acetic acid are produced as by-products.
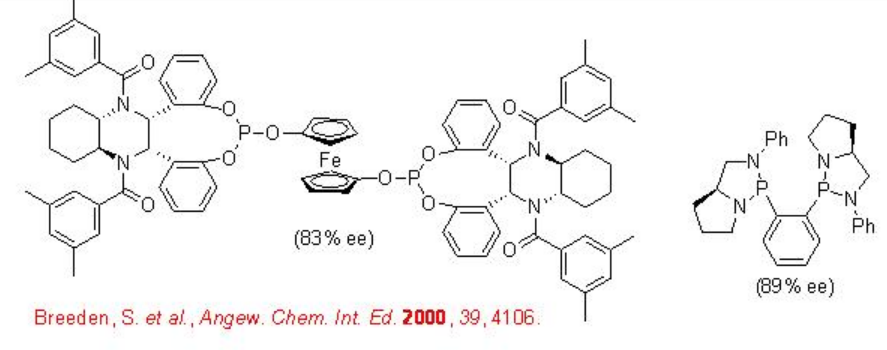
Scheme \(\PageIndex{6}\) illustrates some of the successful ligands for the Rh-catalyzed hydroformylation of vinyl acetate. The enantioselectivity of the reactions are shown in the brackets.
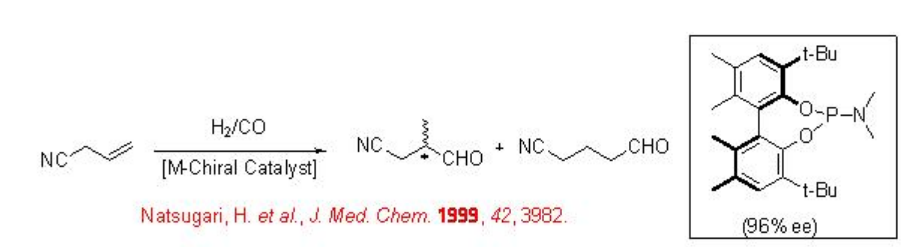
9.1.3 Reaction of Allyl Cyanide
The asymmetric hydroformylation of allyl cyanide is of great interest because the iso-aldehyde derivative can be converted into 2-methyl-4-butanol, which is intermediate, for the asymmetric synthesis of tachikinin, a novel NK1 receptor agonist (Scheme \(\PageIndex{7}\)). The reaction has been studied using diphosphite, phosphine-phosphite, bis-phosphacyclic and phosphoroamidite ligands with up to 96% ee.
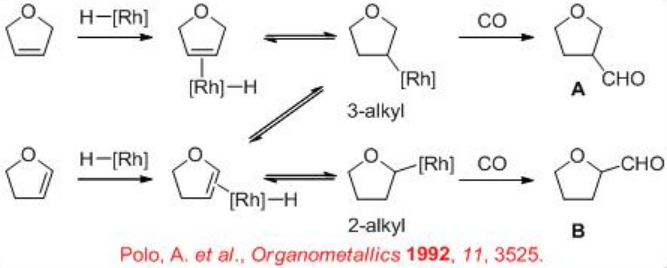
9.1.4 Reaction of Heterocyclic Alkenes
Few studies are focused on the hydroformylation of heterocyclic alkenes. For these substrates, the regioselectivity is of special interest because it is different from that of the acyclic alkenes. For example, the hydroformylation of 2,5-dihydrofuran can lead to the formation of both the tetrahydrofuran-3-carbaldehyde A (expected product) and tetrahydrofuran-2-carbaldehyde B (could be formed via an isomerization process). The regioselectivity is to be controlled by the modification of the ligands and reaction conditions.
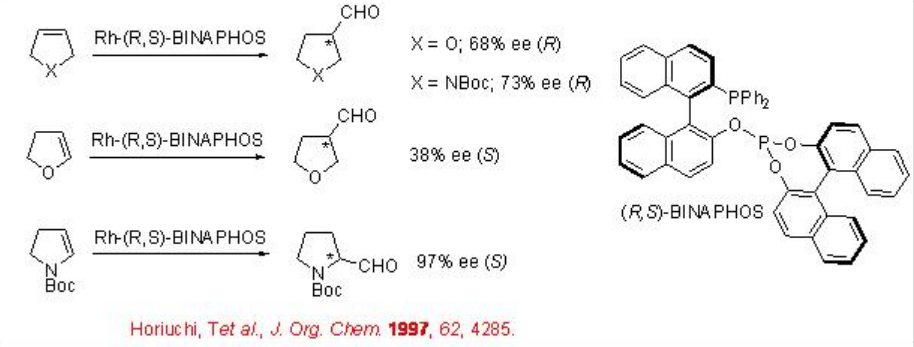
Scheme \(\PageIndex{9}\) summarizes the reaction of 2,5-dihdyrofuran, 3-pyrroline derivative and 4,7-dihdyro-1,3-dioxepin derive using chiral Rh-complex bearing R,S-BINAPHOS. The optically active aldehydes are obtained as single products with enantioselectivities between 64-97%. In case of 2,5-dihydrofuran, up to 64% regioselectivity is observed for the formation of tetrahydrofuran-3-carbaldehyde A , while the reaction of 2,3-dihyrofuran led to the formation of a mixture of A and B (1:1) with an ee of 38% in A.
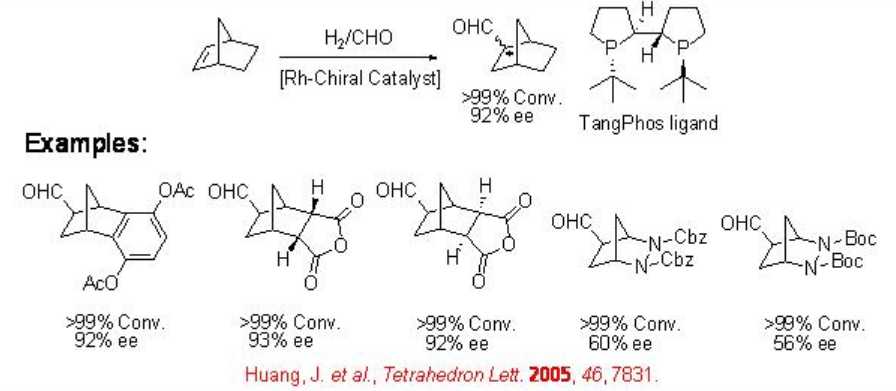
9.1.5 Reaction of Bicyclic Alkenes
The asymmetric hydroformylation of bicyclic alkenes has received little attention. This reaction is interesting because of the following features: (i) the reaction can lead to the formation of three chiral centers upon one C-C bond formation; (ii) there is no regioselectivity problem; (iii) functional groups located opposite to the carbon-carbon double bond could be versatile. Scheme \(\PageIndex{10}\) summarizes some of the examples for the asymmetric hydroformylation of bicyclic alkenes employing Rh-TangPhos.