8.2: Hydroboration, Hydroalumination and Hydrostannation of Alkenes
- Page ID
- 168817
8.2.1 Hydroboration of Alkenes
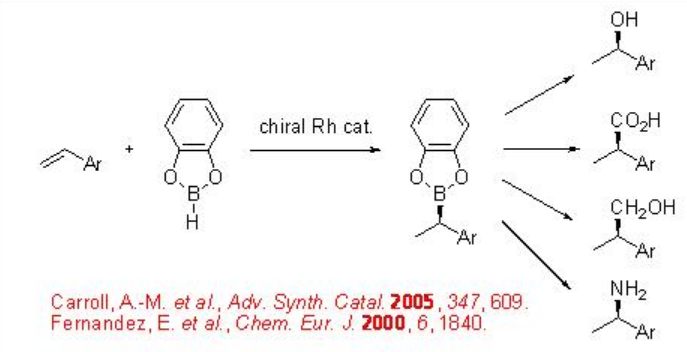
Chiral Rh catalyzed hydroboration of alkenes provides effective method for the synthesis of optically active organoboranes, which are versatile intermediates in organic synthesis. The carbon-boron bond can be converted into several functional group by subsequent carbon-carbon, carbon-oxygen, boron-carbon or carbon-nitrogen bond-forming reactions with retention of stereochemistry (Scheme \(\PageIndex{1}\)).
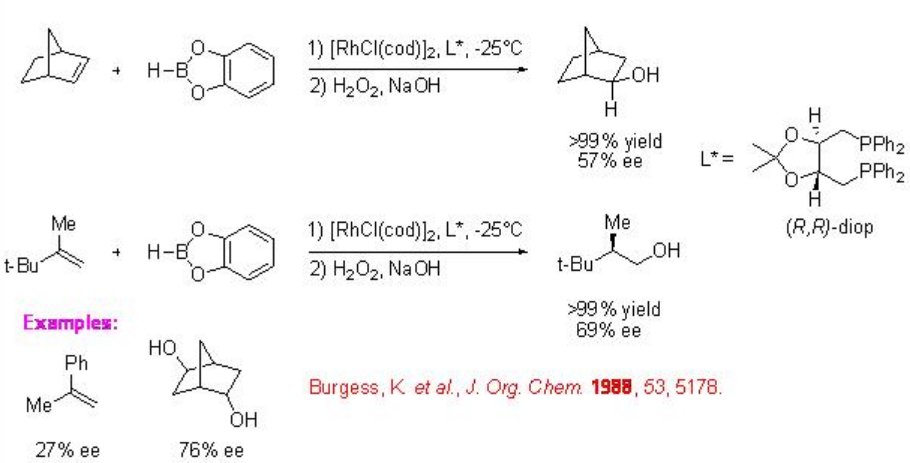
The first catalytic asymmetric hydroboration of norbornene and 2-tert-butylpropene with catecholborane appeared in the presence of Rh-(R, R)-diop complex (Scheme 2). The products, 2-hydroxynorbornane and 2,3,3-trimethylbutanol are obtained after the treatment with alkaline hydrogen peroxide solution.
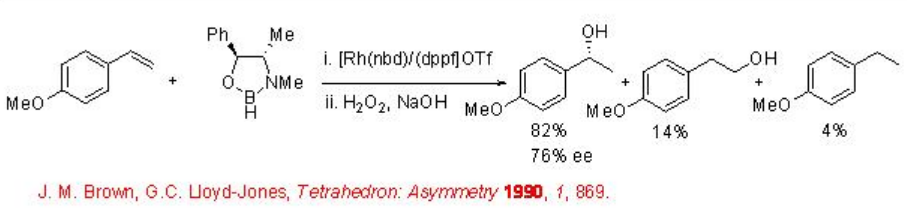
The use of the combination of chiral borane and achiral catalyst has been demonstrated for the asymmetric hydroboration. For example, the hydroboration of 4-methoxystyrene proceeds with chiral borane derived from pseudoephedrine in the presence of achiral rhodium complex to the corresponding secondary alcohol with 76% ee after the oxidation (Scheme \(\PageIndex{3}\)).
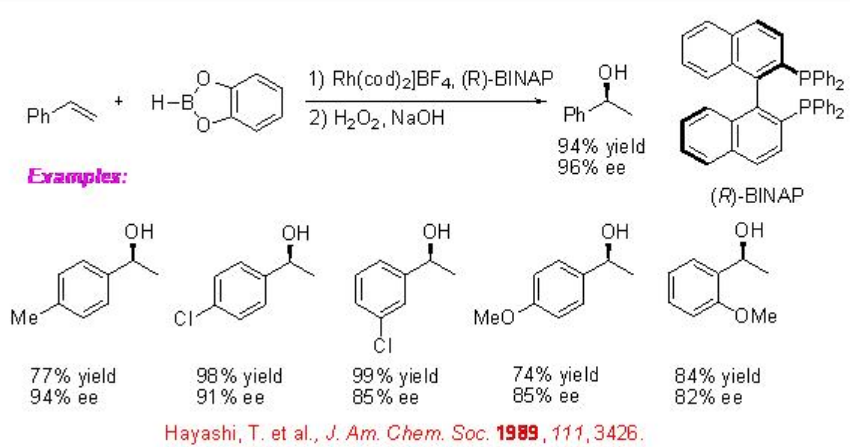
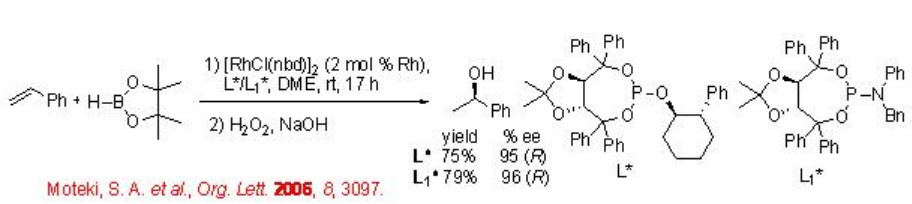
The reaction of vinylarenes with catecholborane has been extensively studied using chiral Rh complex. For example, the cationic Rh-(R)-BIANP catalyzes the hydroboration of styrene with complete branch selectivity to afford 1-phenylethanol with 96% ee after oxidation. The regioselectivity is opposite to that observed with uncatalyzed reactions (Scheme \(\PageIndex{4}\)).
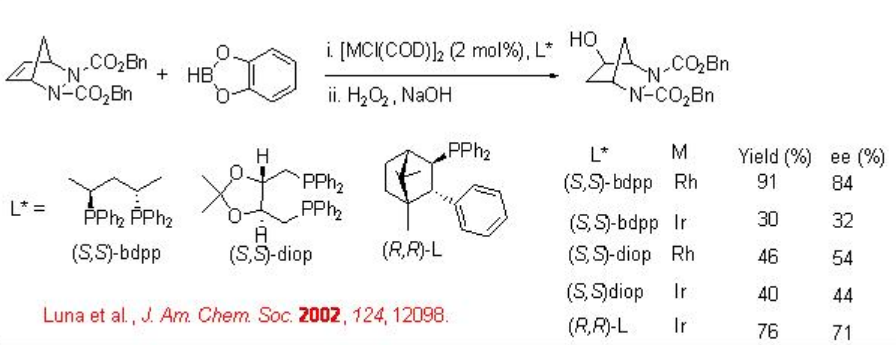
Asymmetric desymmetrization of meso -bicyclic hydrazines has been shown with catecholborane using chiral Rh and Ir-based complexes (Scheme \(\PageIndex{5}\)). A reversal of enantioselectivity is observed between the Rh and Ir catalysts.
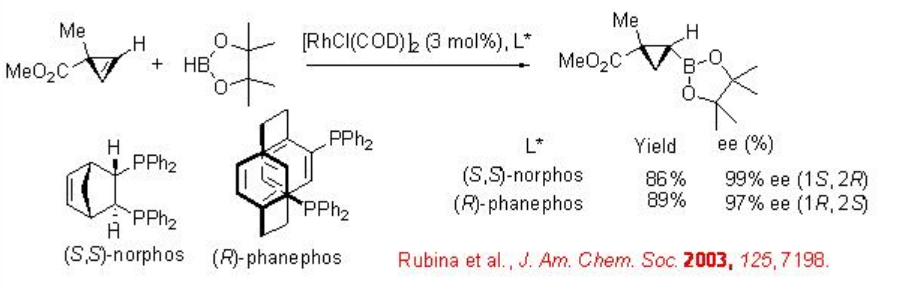
The reaction of cyclopropene is studied with pinacolborane as a new hydroborating agent in the presence of a series of chiral Rh phosphine complexes (Scheme \(\PageIndex{6}\)). The reaction using pinacolborane showed enhanced selectivity compared to that with catecholborane due to steric control between the substrates and the hydroborating agent.
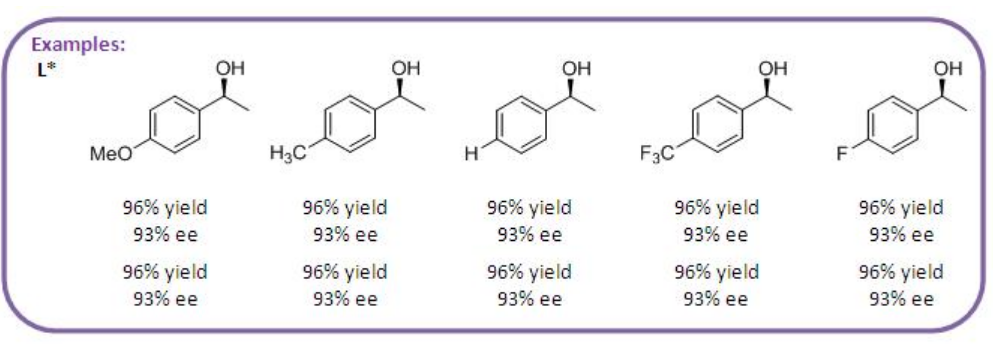
Rh complexes with chiral monodentate phosphate and phosphoramidite derived from taddol are studied for the hydroboration of vinylarenes with pinacolborane (Scheme \(\PageIndex{7}\)). The reactions of a series of vinylarenes having electron withdrawing- and donating substituted proceed with high enantioselectivity.
8.2.2 Hydroalumination and Hydrostannation of Alkenes
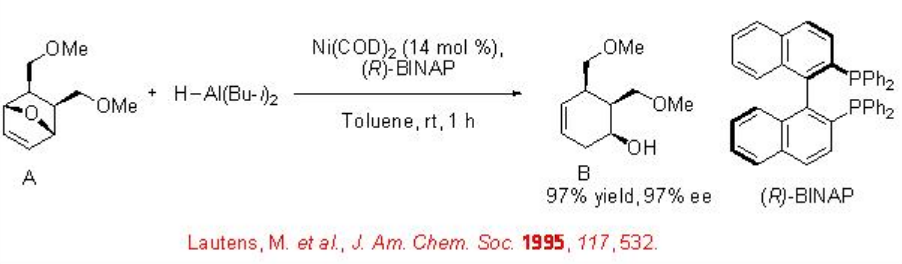
While the catalytic asymmetric hydrosilylation and hydroboration reactions are well known, the catalytic hydroalumination and hydrostannation of alkenes are rare. Chiral nickel complex is used for the asymmetric hydroalumination of oxabicyclic alkenes. For example, Ni-( R )-BINAP catalyzes the reaction of A with iso -Bu2 AlH to give B with 97% ee (Scheme \(\PageIndex{8}\)).
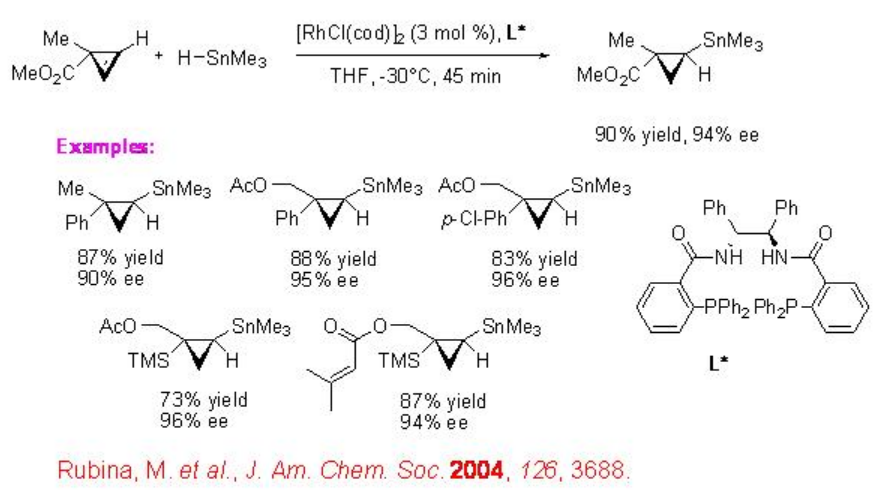
The first example for the asymmetric hydrostannation of cyclopropenes is appeared using Rh-complex bearing chiral diphenylphosphinobenzoic acid-derived L* (Scheme \(\PageIndex{9}\)). The product trans- cyclopropylstannane is obtained with 94% ee. The procedure is general and the reaction a series of substituted cyclopropenes is demonstrated.


