7.5: Microwave-Assisted Reactions
- Page ID
- 171996
The use of microwave irradiation can reduce the reaction time compared to the conventional heating. Thus, sometimes, the side reactions can be minimized with increase of the product yield. Thus, microwave assisted organic synthesis has been widely accepted in academia as well as pharmaceutical industries.
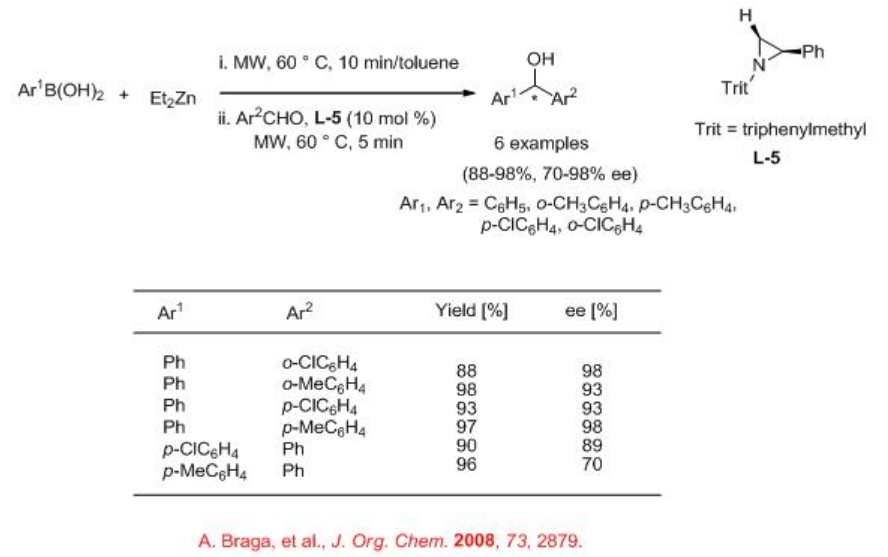
Asymmetric allylic alkyaltions can be effective using chiral molybdenum complex under microwave irradiation in THF (Scheme \(\PageIndex{1}\)). For example, carbonate reacts with dimethyl malonate with high enantioselectivity. Under these conditions, palladium based system gives different regioisomer.
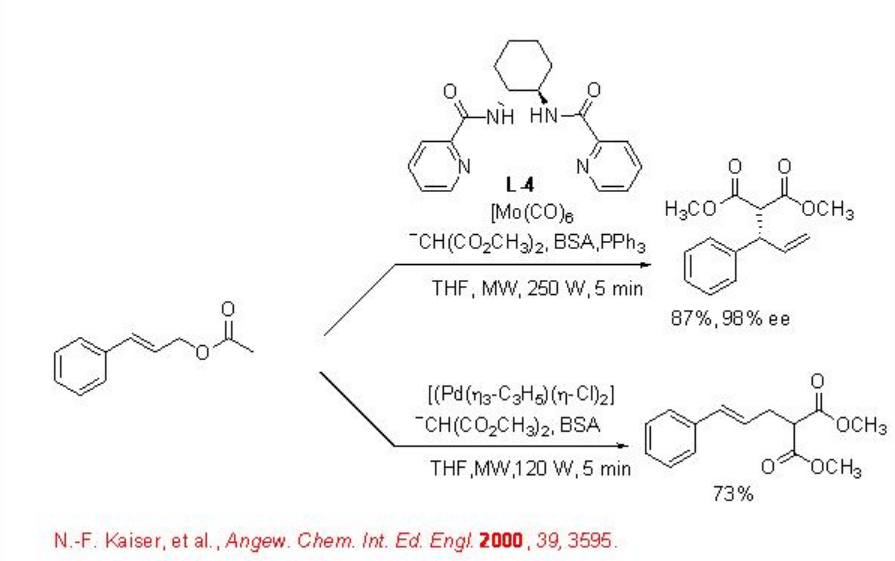
Microwave irradiation has also been found to be effective for the arylation of aromatic aldehydes with high enantioselectivity (Scheme \(\PageIndex{2}\)). For example, the reaction of arylboronic acid with aryl aldehydes in the presence of diethylzinc and aziridine based ligand L5 gives arylated product with up to 98% ee. The reaction time can be decreased from 1 h to 15 min by changing conventional heating to microwave irradiation.