7.3: Reactions in Supercritical Fluids (SCFs)
- Page ID
- 168811
SCF is a substance above its critical temperature (Tc) and pressure (Pc), but below the pressure condensation leads to a solid. At the critical point, high temperature and pressure, the substance can exit both as a vapor and a liquid in equilibrium. Thus, in a closed system, as both the temperature and pressure increase, the liquid becomes less dense due to thermal expansion, and the gas becomes dense as the pressure rises. Thus, the densities of both the phases converge until they become identical at the critical point. At this point, both the phases become indistinguishable and SCF is formed. In such SCF, a high reactivity and selectivity are sometime observed.
Supercritical carbon dioxide (scCO2) offers the advantages that simple depressurization leads to removal of the residual scCO2, and thus, no hazardous solvent is produced, providing effective route for the separation of the products. Thus the synthesis of organic compounds can be accomplished under solvent-free conditions that find wide applications in pharmaceutical, food and cosmetic industries.
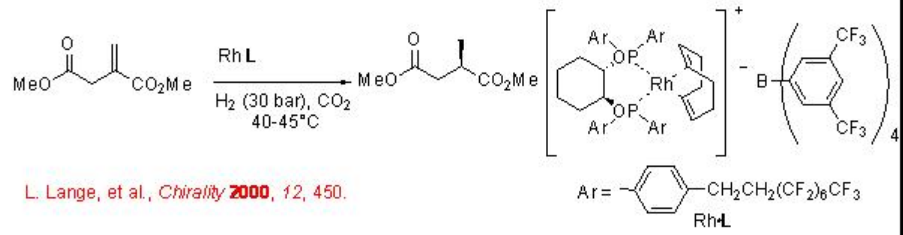
7.3.1 Hydrogenation of Alkenes
Asymmetric hydrogenation of alkenes can be carried out in scCO2 using chiral Rh complex with good enantioselectivity (Scheme \(\PageIndex{1}\)). The catalyst is dissolved in scCO2 during the reaction making the process homogeneous.
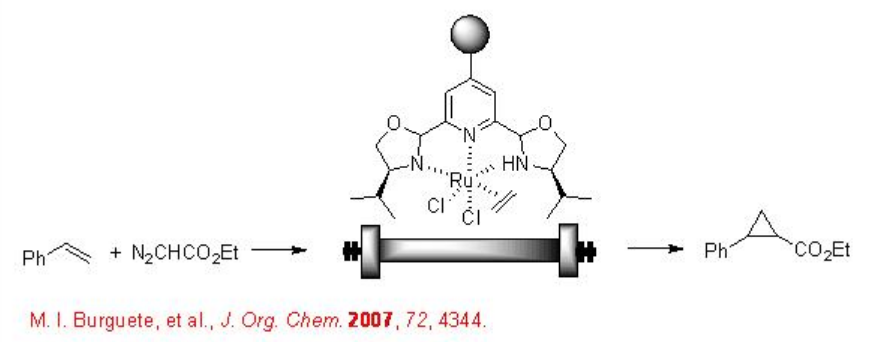
7.3.2 Cyclopropanation
The continuous flow of scCO2 has been used for the asymmetric cyclopropanation using an immobilized chiral Ru complex (Scheme \(\PageIndex{2}\)). The reaction has been reported 7.7 fold more efficient compared to that within dichloromethane solvent. In addition, easy product separation and environmental friendliness makes the process more attractive.