6.1: Reactions Carbon-Carbon Double Bonds
- Page ID
- 168801
Enantioselective reduction of C=C double bond has important application in the synthesis of many natural products and pharmaceutically important compounds. Scheme \(\PageIndex{1}\) summarizes some of the common successful phosphine based chiral ligands developed for the catalytic asymmetric hydrogenation of alkenes.
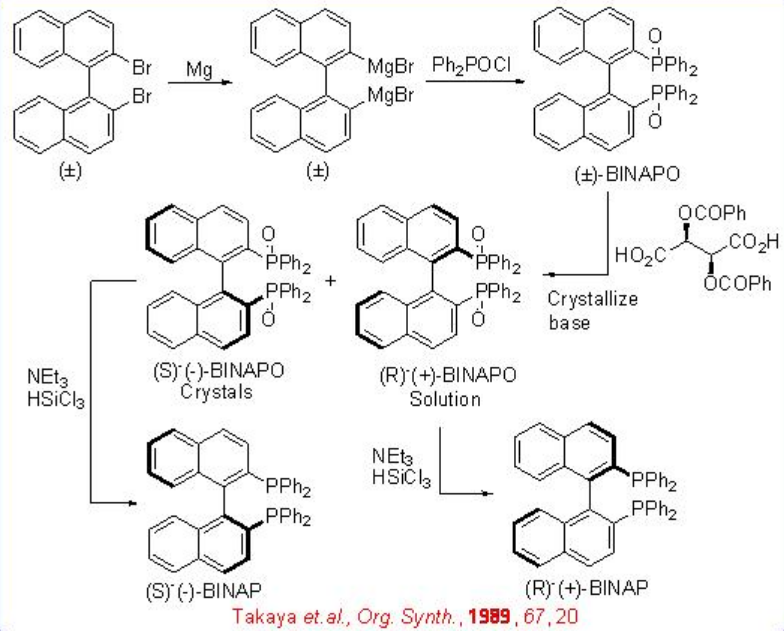
BINAP based ligands play an important role for asymmetric hydrogenation of alkenes. Both (S)- BINAP and (R)- BINAP could be synthesized by resolution methods using (1S,2S)- tartaric acid as well as (8R,9S)- N - benzylcinchonidinium chloride as the chiral sources . Synthesis of (S)- BINAPcould be performed from racemic 2,2'-dibromo BINAP (Scheme \(\PageIndex{2}\)). Resolution of the corresponding phosphine oxide with (1S,2S)- tartaric acid and subsequent reduction with HSiCl3 can afford (S)- BINAP in gram scale.
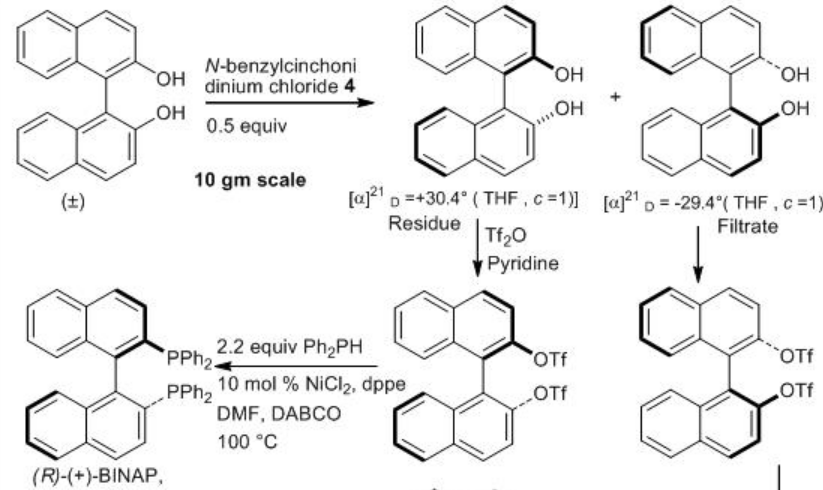
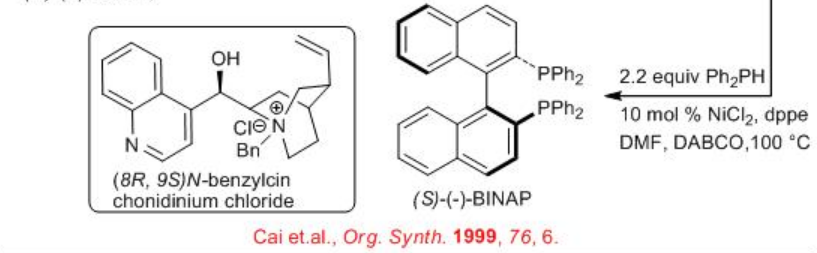
Alternatively, (S)- BINAP and (R)- BINAP can be synthesized by resolution of racemic BINOL using (8R,9S)- N - benzylcinchonidinium chloride (Scheme \(\PageIndex{3}\)) . Converting them into triflate derivative and subsequent cross-coupling with Ph2 PH using NiCl2 to afford (S)- BINAP and ( R ) - BINAP in gram scale. (S)- BINAP ; light brown solid, mp 205°C , 99 % ee, [α]21D= −29.4° ( THF , c =1). (R)- BINAP; white crystalline solid, mp 207°C , 99% ee, [α]21D = 26.2 - 30.9° ( THF , c 1).
6.1.1 Reduction of α,β -Unsaturated Carboxylic acids
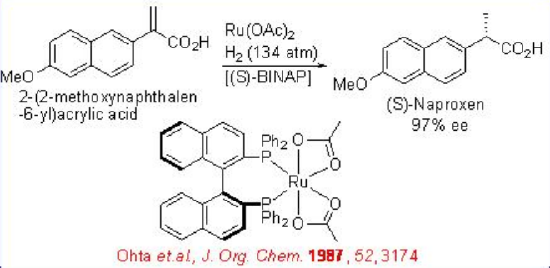
Chiral Ru(II)-BINAP catalyzes the hydrogenation of α,β- unsaturated carboxylic acids. For example, the hydrogenation of naphthacrylic acid can be performed using a Ru-( S )-BINAP with 134 atm H2pressure (Scheme \(\PageIndex{4}\)). The reaction affords chiral ( S )-naproxen with 98% ee, which is a nonsteroidal anti-inflammatory drug.
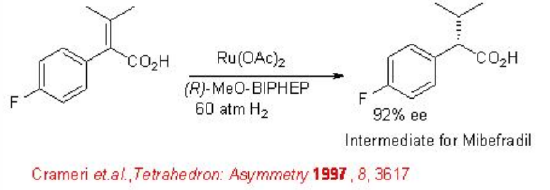
Hydrogenation has been explored for the synthesis of intermediate of (S)- mibefradil. For this reaction chiral Ru-complex bearing ( R )-MeO-BIPHEP is found to be effective affording the target intermediate with 92% ee (Scheme \(\PageIndex{5}\)).
6.1.2 Reduction of Allylic alcohol
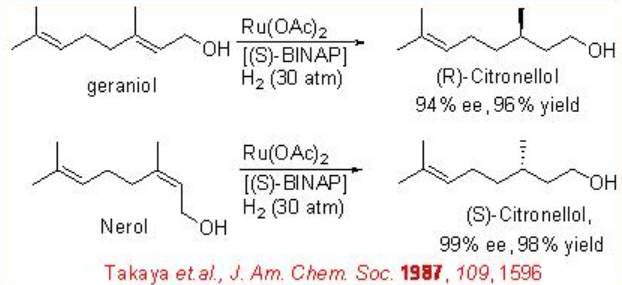
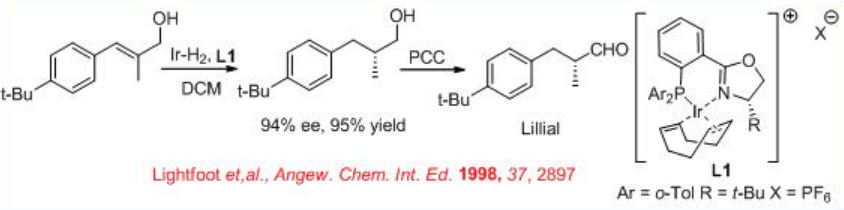
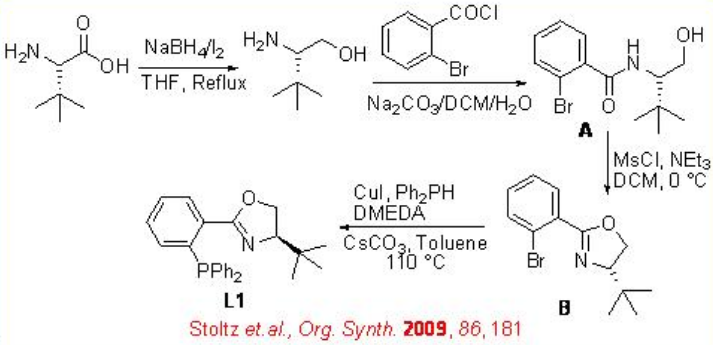
Allylic alcohols can be reduced with high selectivity using chiral Ru-( S )-BINAP as a catalyst. For example, the reduction of geraniol can be accomplished with 94% ee (Scheme \(\PageIndex{6}\)). The reduced product is used for the large scale synthesis of L-(+)-menthol. Under these conditions, nerol undergoes reduction to give ( S )-citronellol in 99% ee. Chiral iridium-based catalytic systems have also been subsequently explored for the asymmetric reduction of allylic alcohols. For example, the complex bearing chiral phosphanodihydrooxazole L1 catalyzes asymmetric reduction of an allyl alcohol, which is used as a key step in the synthesis of lillial (Scheme \(\PageIndex{7}\)). Scheme \(\PageIndex{8}\) illustrates the synthesis of chiral phosphanodihydrooxazole L1.
6.1.3 Reduction of Allylic Amines
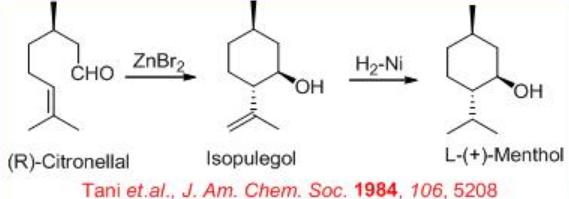
In parallel to the reduction of allylic alcohol, Rh-( S )-BINAP system has been used for the reduction of allylic amine. For example, the synthesis of (R)- citronellal can be accomplished via reduction of allylic amine (Scheme \(\PageIndex{9}\)). The key step is the isomerization of geranyl diethylamine forming (R)-citronellal enamine . The Rh-complex performs the rearrangement of this allylic amine to the enamine creating a new chiral centre with >98% ee, which upon hydrolysis gives (R)-citronellal in 96–99% ee. The latter serves as substrate precursor for the synthesis of L-(+)-menthol via intramolecular ene reaction followed by hydrogenation (Scheme \(\PageIndex{10}\)).

6.1.4 Reduction of α, β-Unsaturated Aldehydes
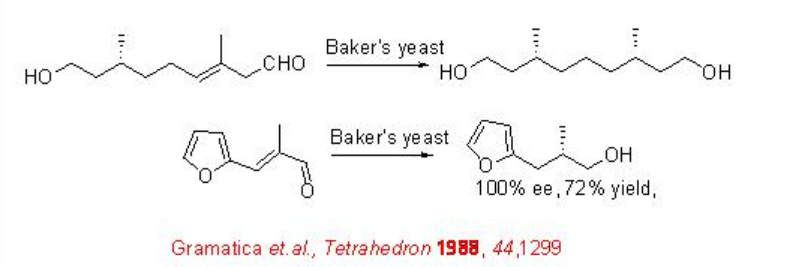
Asymmetric reduction of α, β -unsaturated aldehydes with transition metal catalysts has not yet proven ready for wide spread industrial application. In comparison to CBS catalyst, the Baker's yeast is most useful, since the precursor (R)-proline used to synthesize CBS is expensive. The chiral reduction of enals to chiral alcohols using Baker's yeast has been known for over 30 years. Scheme \(\PageIndex{11}\) summarizes some of the examples for the Baker yeast catalyzed reduction of C=C of α, β -unsaturated aldehydes.
Subsequently, organocatalysis has been found be effective for the asymmetric reduction. A recent interesting development is the organocatalytic hydride transfer reductions of α, β -unsaturated aldehydes to chiral aldehyde. Hantzsch ester acts as a good NADH mimic in the hydride transfer to an iminium ion, formed when the α,β -unsaturated aldehyde reacts with the amine of the organocatalyst (Scheme \(\PageIndex{12}\)).
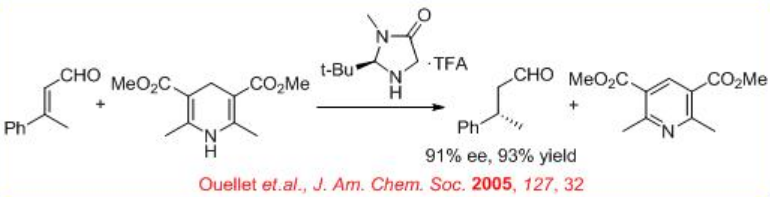
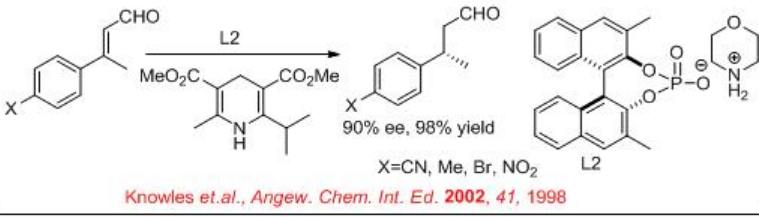
Similarly, chiral phosphoric acid L2 catalyses the reduction of C=C of α, β -unsaturated aldehyde with 90% ee and 98% yield in the presence of Hantzsch ester (Scheme \(\PageIndex{13}\)).
6.1.5 Reduction of α, β-Unsaturated α-Amino Acid
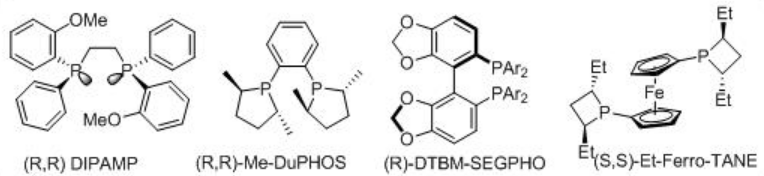
Asymmetric reduction of α, β -unsaturated α-amino acid has wide application in organic synthesis. Chiral biphosphines in combination with Rh acts as the best combination for the reduction α, β-unsaturated α -amino acids. Scheme \(\PageIndex{14}\) summarizes some of the successful chiral phosphines for the Rh-catalyzed reactions.
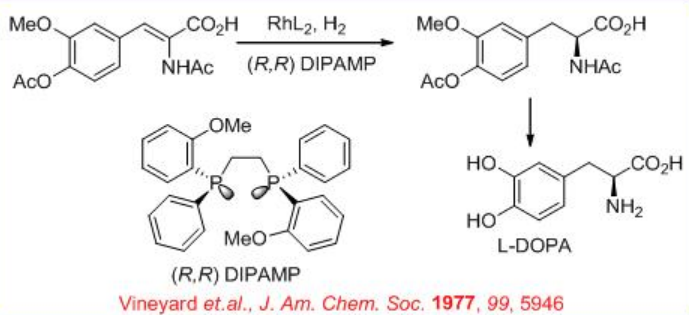
Rh-DIPAMP has been explored for the reduction of α, β-unsaturated α-amino acids. For example, L-DOPA, a chiral drug for treating Parkinson's disease, is synthesized using Rh-( R,R )-DIPAMP catalyzed reduction of α, β-unsaturated α -amino acid as a key step (Scheme \(\PageIndex{15}\)).
Rh -(R,R)- DuPHOS can be used for the reduction of α, β-unsaturated α-amino acid to give chiral amino acid (Scheme \(\PageIndex{16}\)). Using this procedure many of the unnatural α-amino acids can be obtained directly with enantioselectivity approaching 100% ee and S/C ratio 10000-50000. The rhodium-catalyzed hydrogenation of the E- and Z -isomers, with BINAP in THF, affords products with opposite absolute configurations. Remarkably, the (R,R)- DuPHOS system provides excellent enantioselectivity for both isomeric substrates with the same absolute configuration, irrespective of the E/Z -geometry. This result is particularly important for the construction of alkyl dehydroamino acid derivatives, which are difficult to prepare in enantiomerically pure form.
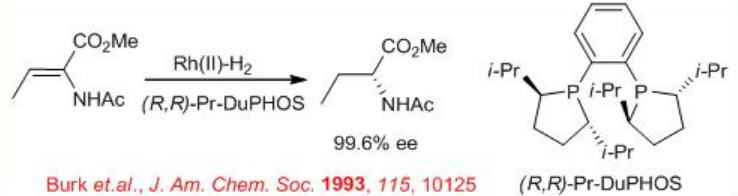
The hydrogenation of the ( E)- or ( Z)- isomer of β-(acetylamino)- β-methyl- α-dehydroamino acids with Rh(I)-Me-DuPHOS provides either diastereomers of the N, N -protected 2,3-diaminobutanoic acid derivatives with 98% ee (Scheme \(\PageIndex{17}\)-\(\PageIndex{18}\)).
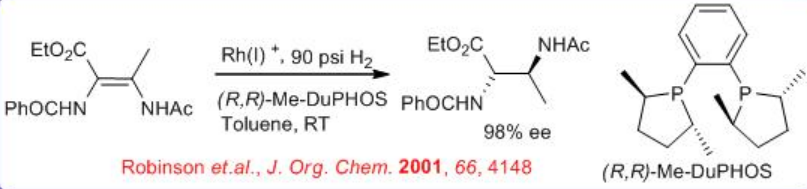
(S)- SEGPHOS and its analogous provide superior results in Ru-catalyzed hydrogenation of four and five-membered cyclic lactones or carbonates bearing an exocyclic methylene group. For example, the reduction of the four membered lactone can be achieved with excellent enantioselectivity using S/C=12270 (Scheme \(\PageIndex{19}\)).
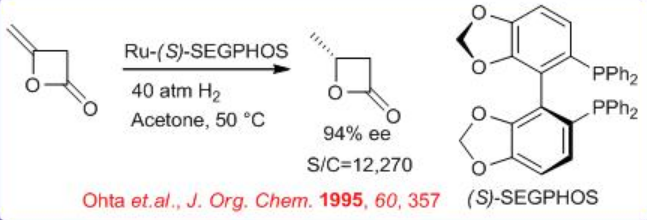
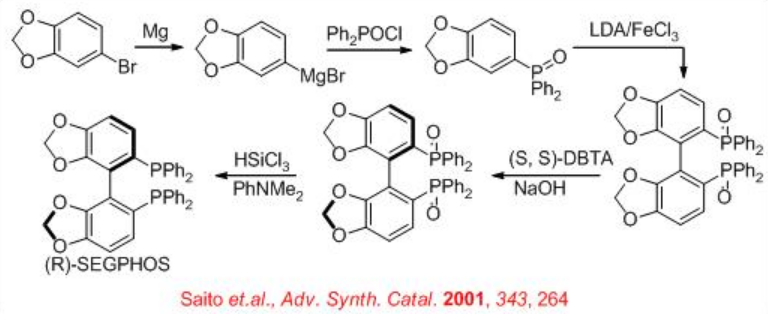
Scheme \(\PageIndex{20}\) describes the synthesis of SEGPHOS. The key step is the resolution of racemic phosphine oxide with (S,S)- DBTA (di-benzoyl-tartaric acid) to provide chiral phosphine oxide. Subsequent reduction with HSiCl3 affords the target SEGPHOS in good yield.
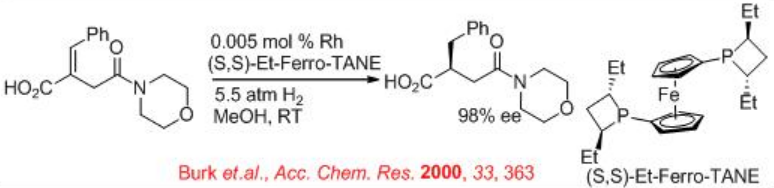
Moreover, chiral 1,10-diphosphetanylferrocene Et-FerroTANE serves as an effective ligand for the rhodium-catalyzed hydrogenation of β -aryl- and β -alkyl-substituted monoamido itaconate (Scheme \(\PageIndex{21}\)). For example, Et-DuPHOS–Rh is utilized for the asymmetric hydrogenation of the trisubstituted alkene to afford the reduced product, which is used for synthesis of intermediate of the drug candoxatril in 99% ee . Candoxatril is the orally active prodrug of candoxatril (UK-73967) human neutral endopeptidase (Neprilysin).
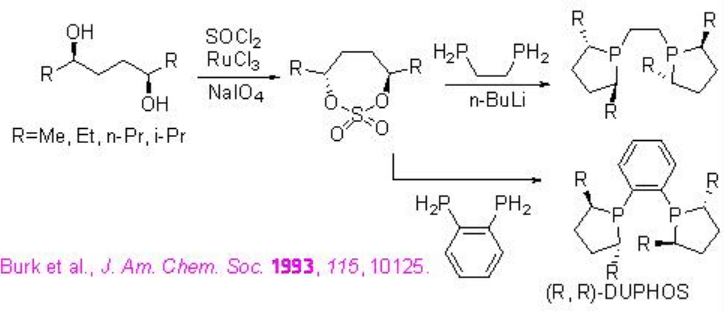
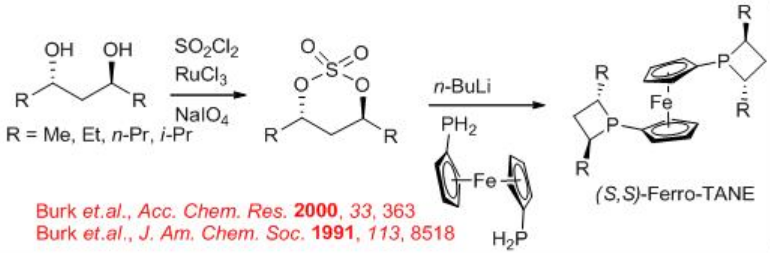
The above described alkyl/aryl-ferro-TANE family ligands could be synthesized from optically active diols (Scheme \(\PageIndex{22}\)). Cyclization with SO2Cl2 in presence of RuCl3 and NaIO4 affords chiral cyclized sulfonate, which reacts with ferro-phosphine in the presence of n-BuLi to give the target chiral alkyl/aryl-Ferro-TANE family in good yield.
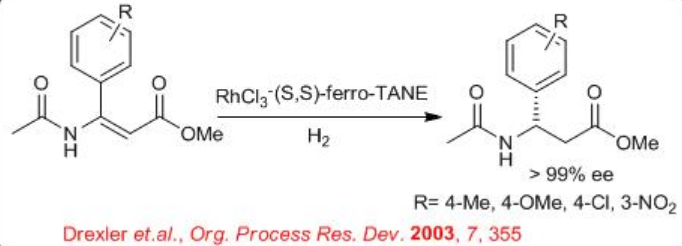
Similarly, the reduction of α,α -disubstituted α, β-unsaturated ester can be carried out using chiral Ru-Et-Ferro TANE (Scheme \(\PageIndex{23}\)). The reaction is compatible with different electron donating and withdrawing groups attached to benzene ring.
6.1.6 Reduction of α -Alkyl Substituted Acids
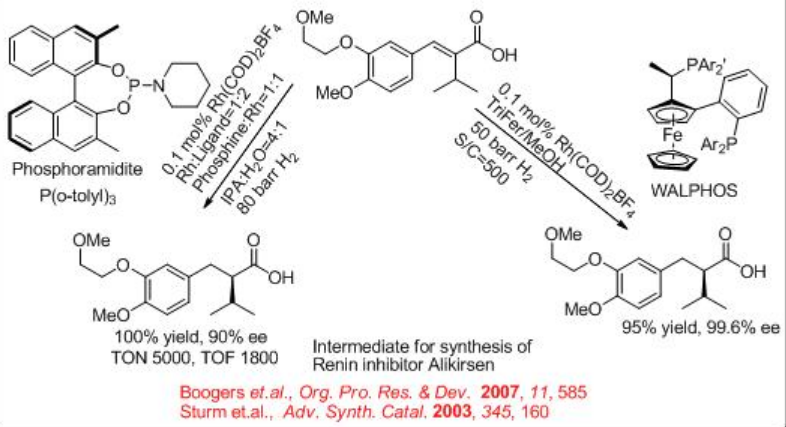
Another important chiral acid is the α -alkyl substituted acid which is used in the synthesis of aliskiren (the active ingredient of Tekturna1) (Scheme \(\PageIndex{24}\)). The key step for the synthesis requires the hydrogenation of cinnamic acid derivative in the presence of Rh-phosphoramidite . The reduction also affords 97% ee using Rh-WALPHOS.
6.1.7 Reduction of α, β-Unsaturated Nitriles
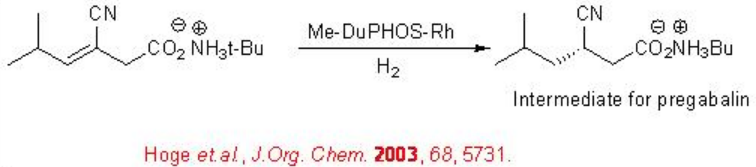
The asymmetric reduction of unsaturated nitriles is a very useful process for the synthesis of many pharmaceutical intermediates. An important application of this strategy involves the further reduction of the nitrile group to yield chiral amines. For example, chiral Rh-phosphine catalyzes the asymmetric hydrogenation of an unsaturated nitrile (Scheme \(\PageIndex{25}\)). The reduced product is used for the synthesis of the Pregabalin.
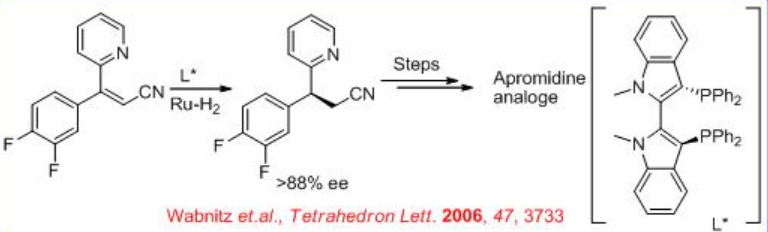
A more challenging example of an unsaturated nitrile reduction that lacks the carboxylate functional group is the asymmetric reduction of the nitrile shown in Scheme \(\PageIndex{26}\). The reduced product is used for the synthesis of chiral 3,3-diarylpropylamine, which is an intermediate for the synthesis of the Arpromidines. The arpromidines analogues are the most potent histamine H2 receptor agonists known and are promising positive inotropic vasodilators for the treatment of severe congestive heart failure.
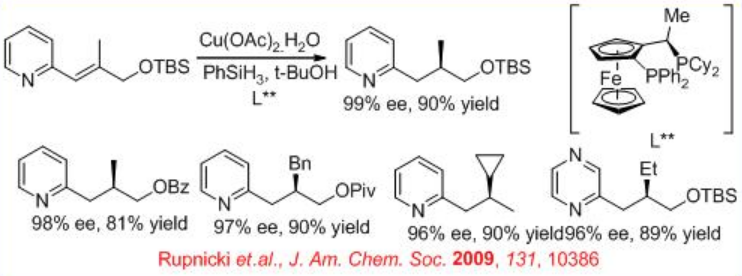
In parallel to Ru, Rh and Ir-based catalytic systems, chiral copper hydride catalysis have been demonstrated for enantioselective 1,4-reductions of 2-alkenyl heteroarenes. Both azoles and azines serve as efficient activating groups for this process (Scheme \(\PageIndex{27}\)).