5.2: Epoxidation of Allylic Alcohols
- Page ID
- 168795
Epoxidation of allylic alcohols is a well developed practical process in asymmetric catalysis.
Titanium-Catalyzed Epoxidation
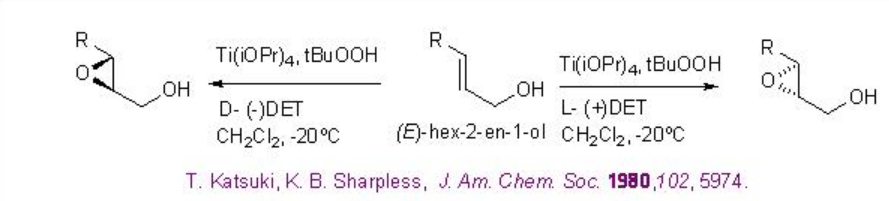
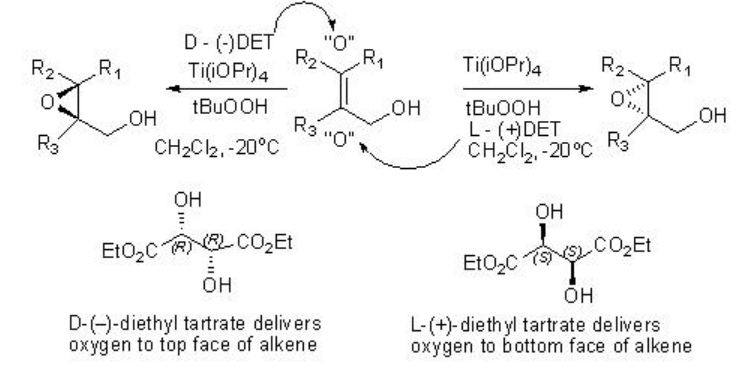
The Sharpless asymmetric epoxidation of allylic alcohol provides a powerful tool for the synthesis of optically active epoxy alcohol. For example, hexe-2-en-1-ol undergoes epoxidation to give chiral epoxy alcohols with 94% ee and 85% yield in presence of 5-10 mol% of Ti(OiPr)4 , L-(+)-DET and t -BuOOH (Scheme \(\PageIndex{1}\)). Using D-(-)-DET as chiral source the opposite enantiomer can be obtained with similar yield and enantioselectivity.
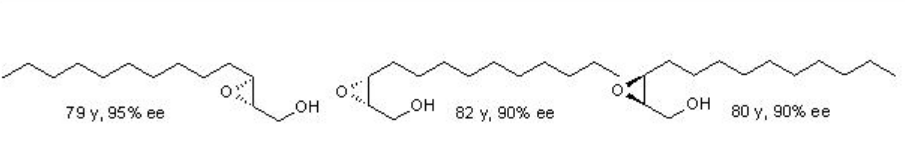
Examples:
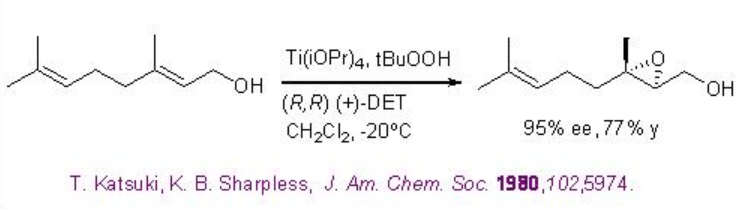
In case the substrates having more double bonds, the allylic double bond can be oxidized. For example, the allylic double bond of geraniol can be selectively oxidized with 95% ee (Scheme \(\PageIndex{2}\)).
Mechanism
The reaction of titanium alkoxide with tartrate ligands leads to the formation of the dimers 1 and 4 that in the presence of t -BuOOH are converted into the intermediates 2 and 5, respectively, by displacement of the isopropoxide and tartrate carbonyl groups (Scheme \(\PageIndex{3}\)-\(\PageIndex{4}\)). Reaction of 2 and 5 with allylic alcohol give the intermediates 3 and 6, respectively. The stereochemistry of the epoxide is determined by the diastereomer of the chiral tartrate diester.
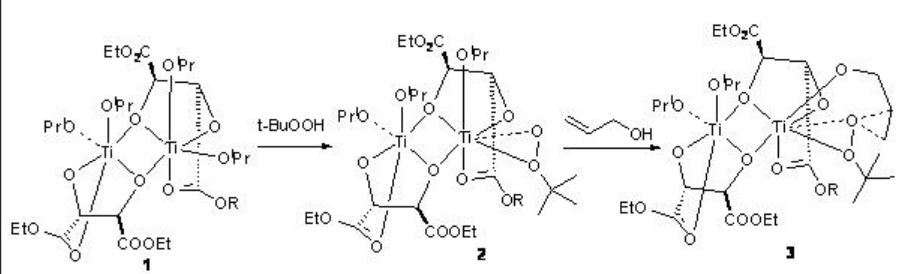
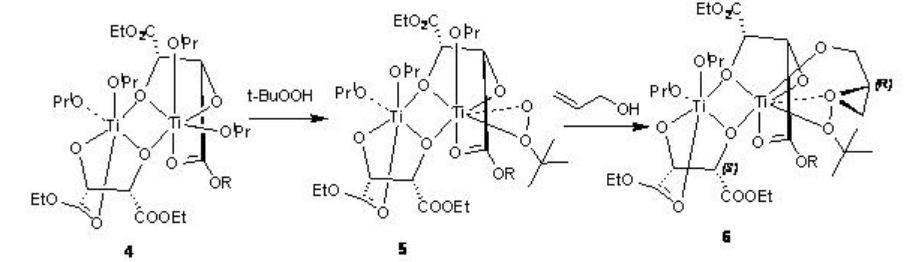
The product stereochemistry can be predicted using the model shown in Scheme \(\PageIndex{5}\).
Application
The reaction has been applied for the synthesis of a number of natural products, antibiotics and pharmaceuticals. For examples, the synthesis of the sex pheromone of gypsy moth (Lymantria dispar) (+)-disparlure 12 has been accomplished (Scheme \(\PageIndex{6}\)). The epoxidation of allyl alcohol 7 by Sharpless procedure affords optically active epoxy alchohol 8 with 95% ee that in presence of pyridinium dichlorochromate (PDC) gives chiral aldehyde 9 . The latter with Wittig salt 10 affords trans -alkene 11 that could be reduced using Pd/C to give the target (+)-disparlure 12 .
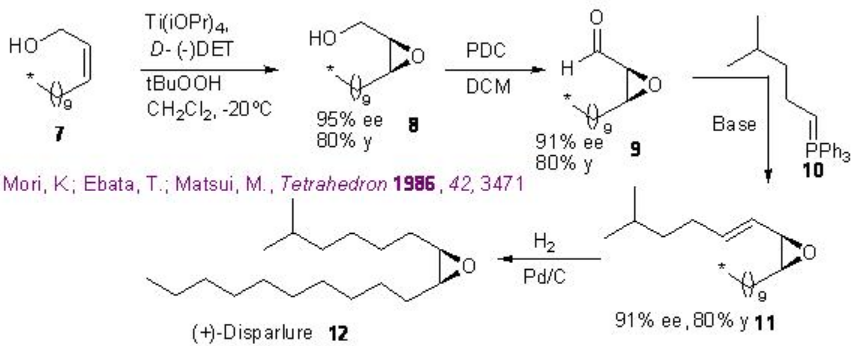
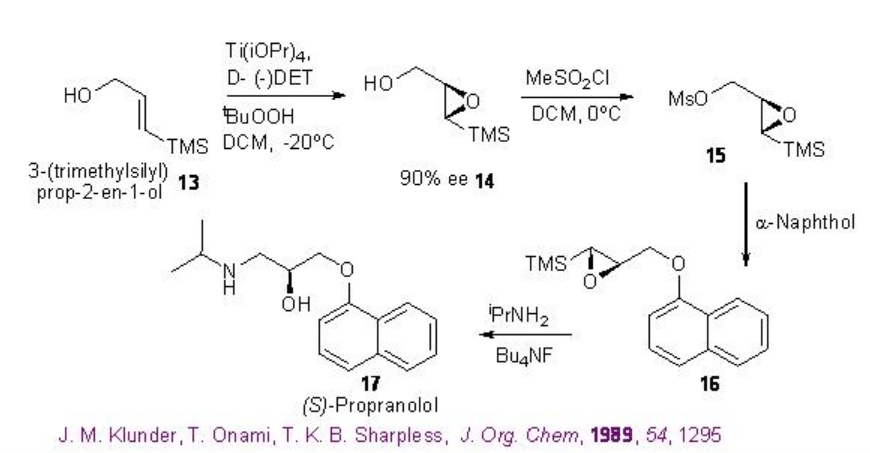
The Scheme \(\PageIndex{7}\) shows the use of the Sharpless asymmetric epoxidation for the synthesis of gastric inhibitor (S) -propanolol. The epoxidation of 3-(trimethylsilyl) prop-2-en-1-ol 13 affords epoxy alcohol 14 with 90% ee that could be converted into 16 by mesylation 15 followed by coupling with 1-naphthol. Opening of the epoxide 16 with isopropylamine leads to the formation of the target (S) -propanolol 17 .
Vanadium-Catalyzed Epoxidation
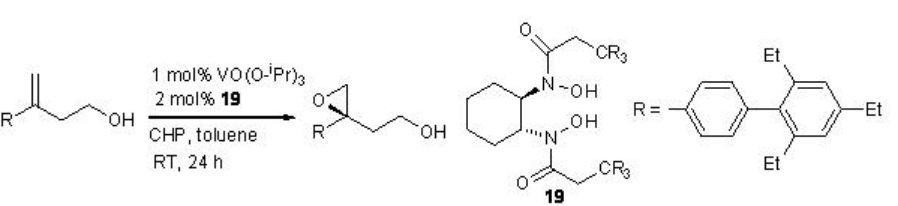
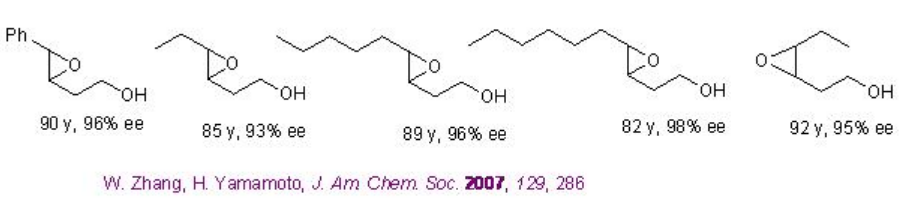
Few Studies are focused on chiral vanadium catalyzed the epoxidation of allylic alcohols. The epoxidation of homoallylic alcohol has been found to be successful (Scheme \(\PageIndex{8}\)).
Examples:
Niobium-Catalyzed Epoxidation
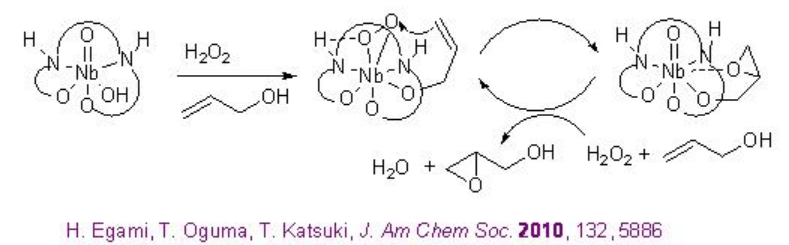
Chiral niobium-complexes catalyze the epoxidation of allylic alcohols in the presence of hydrogen peroxide (H2O2) or urea hydrogen peroxide (UHP). From environmental and economic standpoint, this process is more attractive because it is atom economical and generates water as by-product. For example, [(μ-oxo){Nb(salan)}2] 20 catalyzes the epoxidation of allylic alcohols in the presence of UHP at ambient conditions (Scheme \(\PageIndex{9}\)-\(\PageIndex{10}\)).
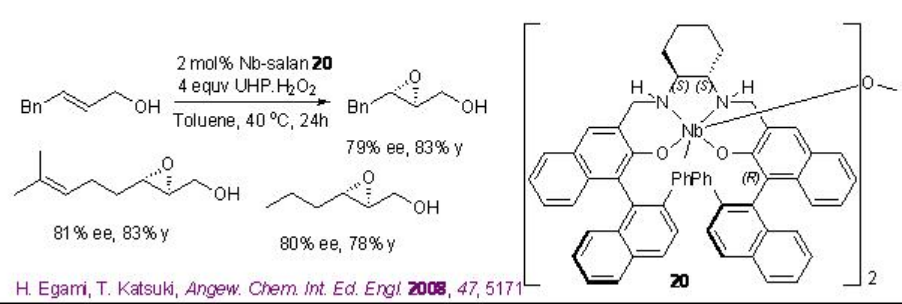
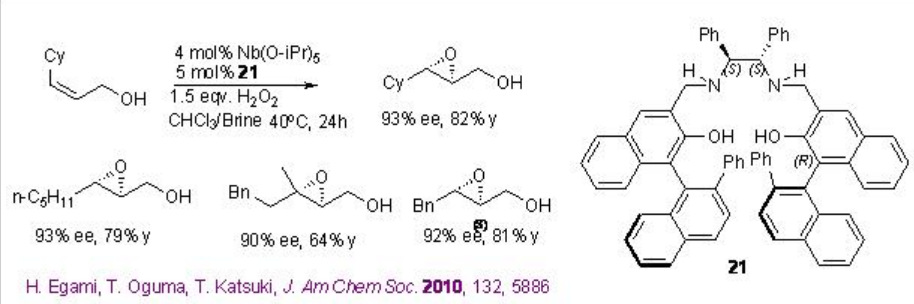
In this protocol, the μ-oxo dimer dissociates into a monomeric species that catalyzes the reaction (Scheme \(\PageIndex{11}\)). Moreover, monomeric Nb(salan) complexes prepared in situ from Nb(OiPr)5 and salan ligands followed by water treatment are found to catalyze the epoxidation better using aq. H2O2with enantioselectivity ranging from 83 to 95% ee. This is the first example of the enantioselective epoxidation of allylic alcohols using aq. H2O2 as terminal oxidant.