4.8: Boration of Alkenes
- Page ID
- 169804
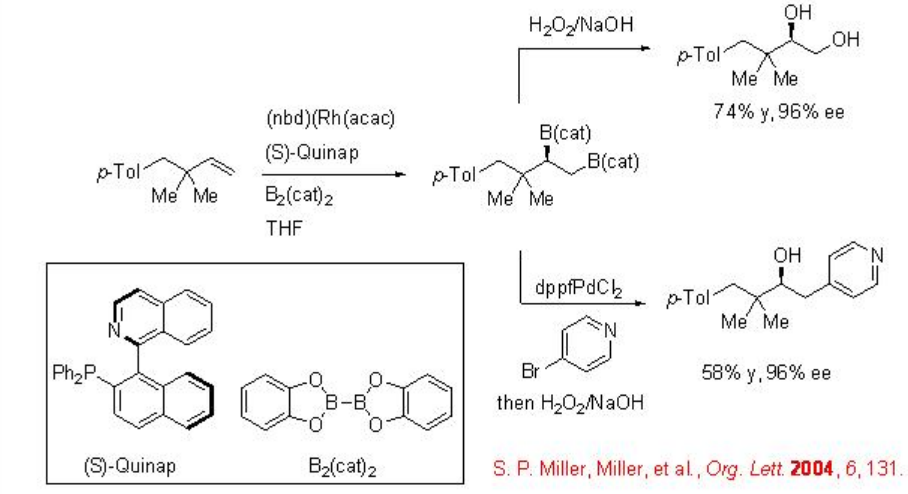
Organoboranes are useful reagents for organic synthesis. Recently, catalytic methods have been developed for enantioselective boration of unsaturated substrates. For example, the diboration of alkenes with bis(catecholato)diboron using rhodium(I) salt and (S)-quinap can be accomplished (Scheme \(\PageIndex{1}\)). Oxidation of the diborane derivatives can lead to chiral 1,2-diols. Furthermore, tandem diboration, Suzuki cross-coupling and oxidation reaction can lead to carbohydroxylation with similar enantioselectivity.
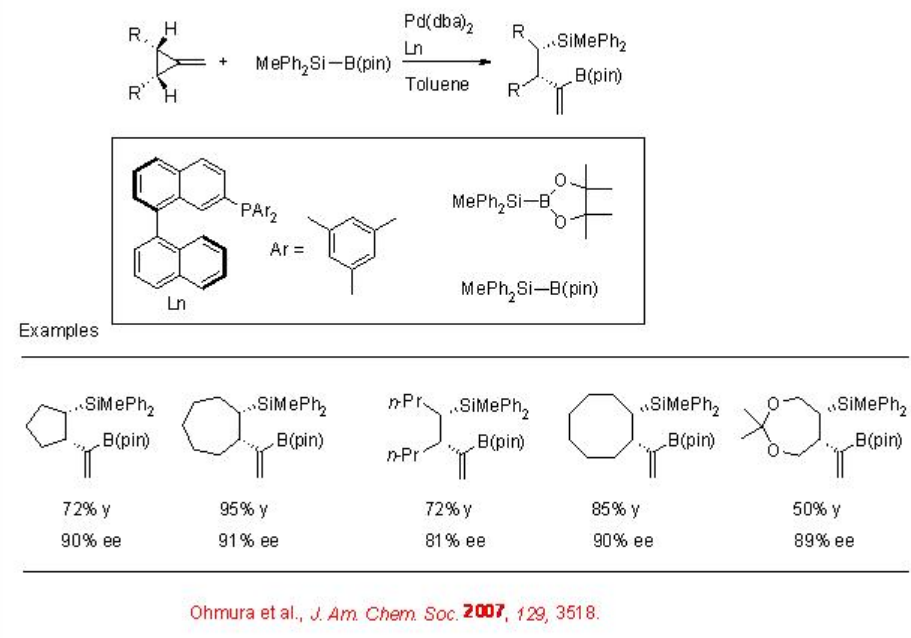
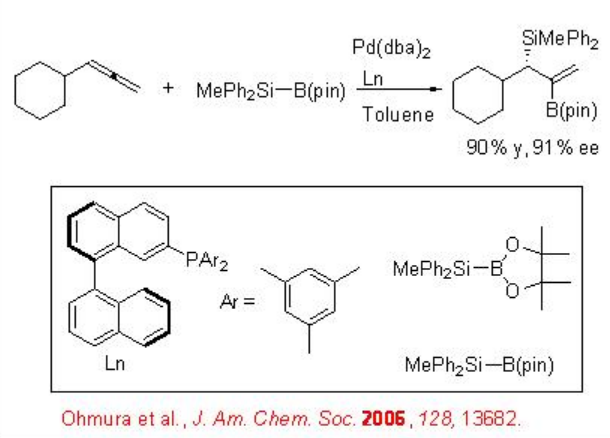
The asymmetric silaboration of symmetrically substituted meso -methylcyclopropanes can be accomplished via carbon-carbon bond cleavage employing chiral palladium-catalyzed boration withMe2PhSiB(pin) as the silylboron reagent (Scheme \(\PageIndex{2}\)). The catalytic system is also effective for the silaboration of mono-substituted allene to give allylsilane with good enantioselectivity (Scheme \(\PageIndex{3}\)).
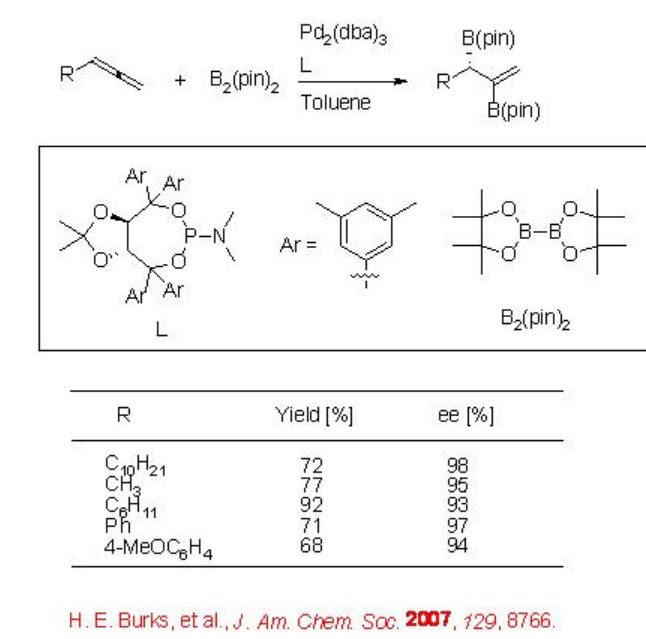
The diboration of terminal allenes is also demonstrated using palladium complex derived from Pd(dba)2 and a chiral phosphoramidite to give 1,2-bis(boronate)ester with high enantioselectivity (Scheme \(\PageIndex{4}\)). The rate determining step involves the oxidative addition of the diboron to Pd, which is followed by the transfer of both boron groups to the unsaturated substrate via a π -allyl complex.