4.7: Amination of Carbonyl Compounds
- Page ID
- 169803
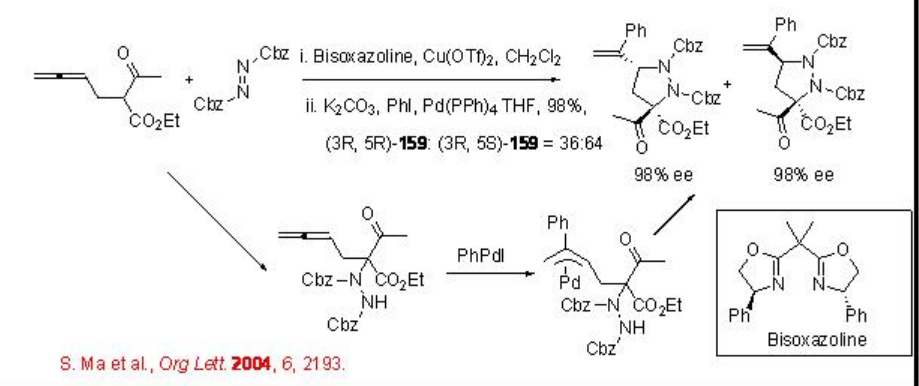
The electrophilic amination reaction is useful technology for the introduction of an amine functionality next to carbonyl carbon. Asymmetric version of this process has been considerably explored. Recently, the use of the combination of copper and palladium based catalytic system has been demonstrated for the asymmetric one-pot tandem addition-cyclization reaction of 2-(2',3'-dienyl)- β-keto esters, aryl halides, and dibenzylazodicarboxylate to afford pyrazolidine (Scheme \(\PageIndex{1}\)). An involvement of π -allylpalladium intermediate via the carbopalladation of allene has been proposed.
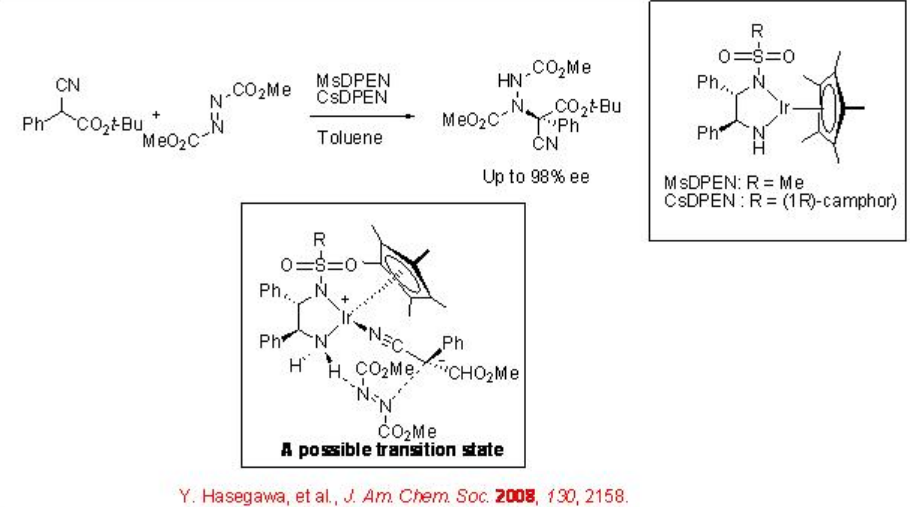
The use of bifunctional chiral amide iridium complex for the direct amination of α -substituted α -cyanoacetate with azodicarboyxlate has been demonstrated with excellent enantioselectivity (Scheme \(\PageIndex{2}\)). In this reaction, the chiral amide complexmay be involved in the deprotonation of cyanoacetate that would lead to the formation of N -bound nitrile complex; thus, cyanoacetate and azodicarboxylate are activated sequentially by the bifunctional catalyst that could facilitate the transformation.
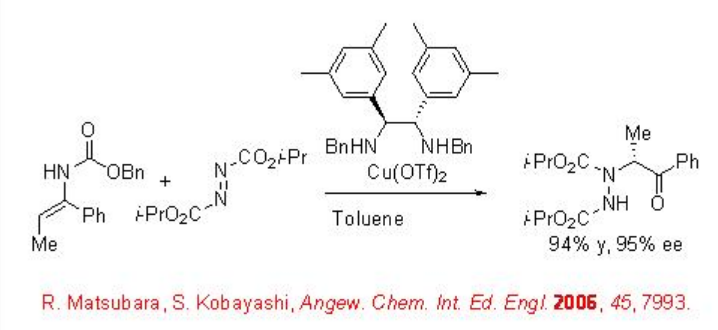
Using chiral diamine-copper(II) the amination of enecarbamates can be accomplished with excellent enantioselectivities (Scheme \(\PageIndex{3}\)). Under these conditions, the changing the enecarbamate geometry from Z to E resulted in a dramatic improvement of the reactivity.