4.6: Aziridination of Alkenes
- Page ID
- 169801
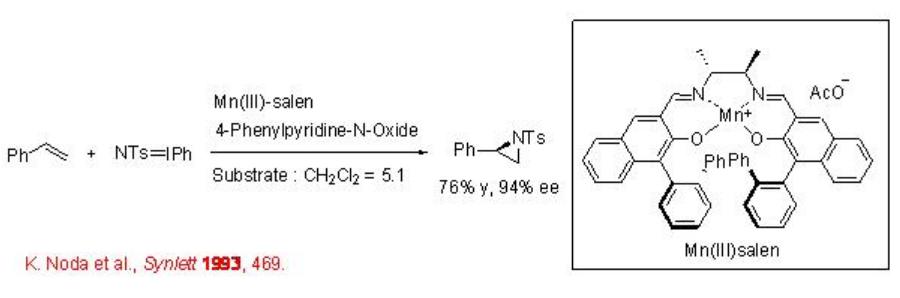
The aziridination of alkenes has been successfully accomplished using chiral Mn-salen with 94% ee. The presence of catalytic amount of 4-phenylpyridine- N -oxide leads to enhancement in the enantioselectivity (Scheme \(\PageIndex{1}\)).
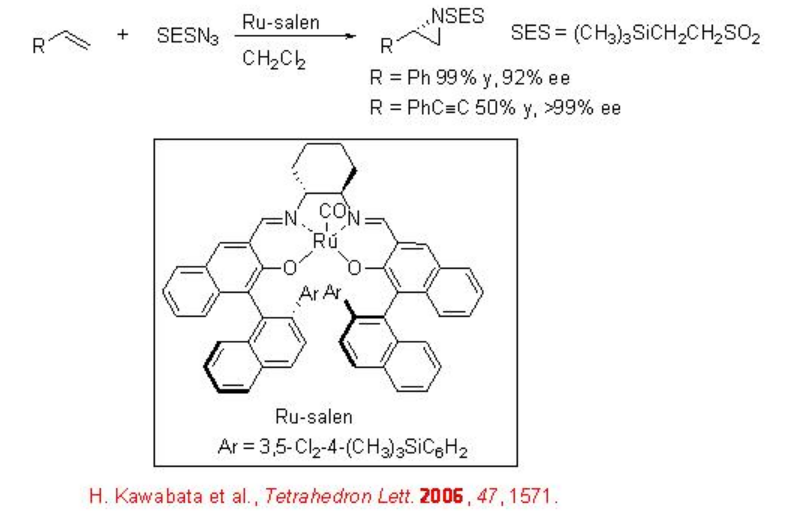
Chiral Ru(salen)(CO) can be utilized for the aziridination using 2-(trimethylsilyl)ethanesulfonyl (SES) group as a nitrene precursor, because the SES group is an easily removable N -protecting group under milder conditions (Scheme \(\PageIndex{2}\)). These reaction conditions are compatible for the reactions of conjugated alkenes with high enantioselectivity.
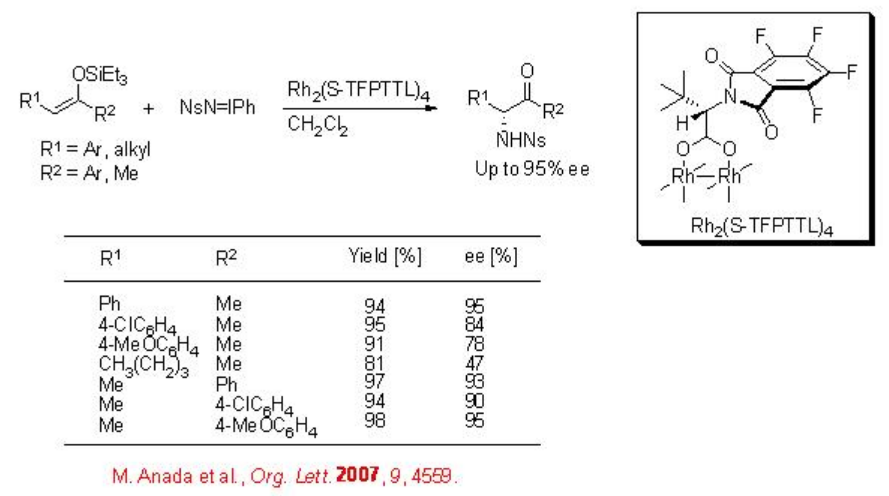
Although the aziridination of alkenes has been explored well, the reaction of enols remains elusive. The aziridination of enols generally lead to α -amino ketones via the ring opening process of the aziridine intermediates. The chiral dirhodium complex, Rh2(S -TFPTTL)4, catalyses efficiently the amination of enol ethers employing NsN=IPh as a nitrogen source (Scheme \(\PageIndex{3}\)). The use of the N-2-nitrophenylsulfonyl (Ns) group is synthetically valuable, because the alkylation and deprotection of N -monosubstituted Ns-amide takes under milder conditions. The application of this protocol has been shown in the formal synthesis of (-)-metazocine.
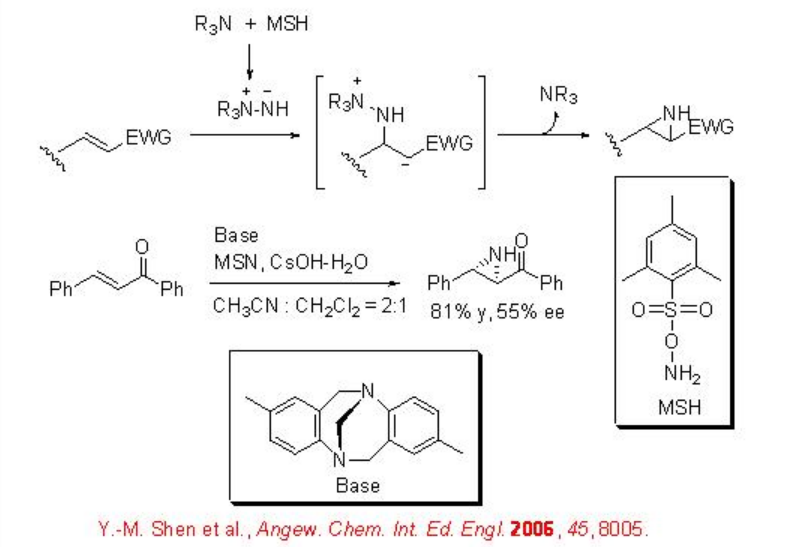
The use of chiral amine has been demonstrated for the reaction of electron deficient alkenes. For example, the use of aminimide as an effective NH-transfer reagent for the aziridination of electron deficient alkenes is reported (Scheme \(\PageIndex{4}\)). In this reaction, in situ generation of a hydrazinium salt from tertiary amine and O-mesitylenesulfonylhydroxylamine (MSH), deprotonation of the hydrazinium salt to form an aminimide, and subsequent aziridination is involved.