4.2: Aza-Claisen Rearrangement and Related Reactions
- Page ID
- 168788
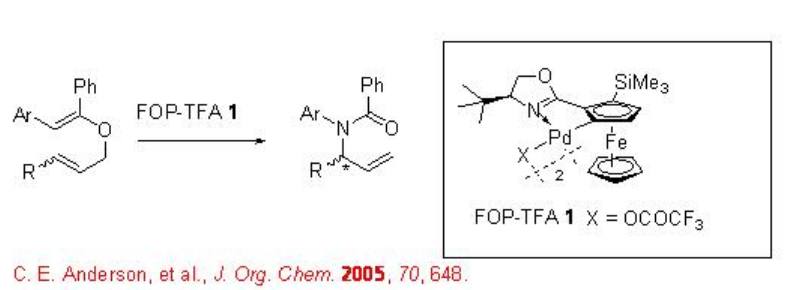
Aza-Claisen rearrangement, known as the Overman rearrangement, has been extensively studied that allows us to synthesize chiral allylic amines from achiral allylic imidates with excellent enantioselectivity. For example, prochiral N -arylbenzimidates can be converted into chiral N- a rylbenzamides in the presence of ferrocenyloxazoline palladacycle , FOP-TFA, (Scheme \(\PageIndex{1}\)).
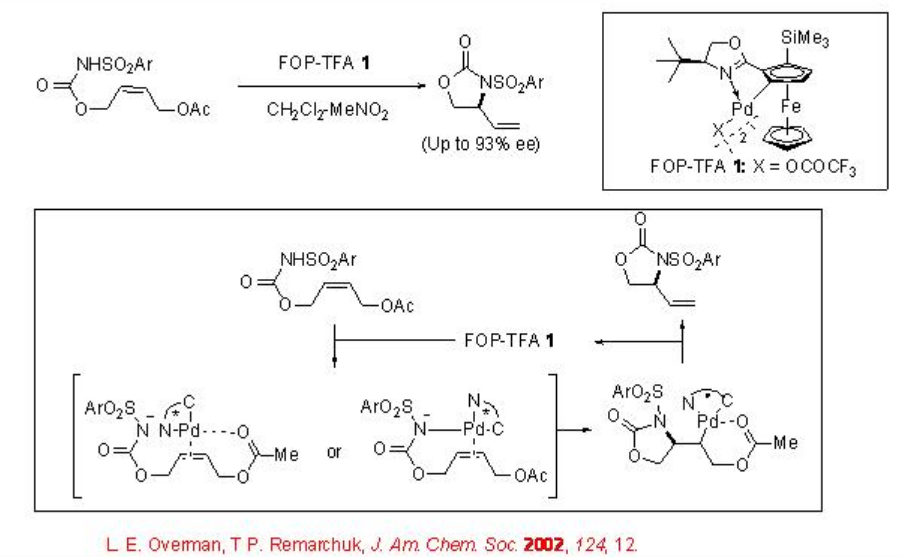
This catalytic system has also been shown to promote the cyclization of allylic N -arylsulfonyl carbamates to give five-membered nitrogen containing heterocycles (Scheme 2). An involvement of aminopalladation of the alkene followed by insertion of the alkene into the Pd-N has been proposed.
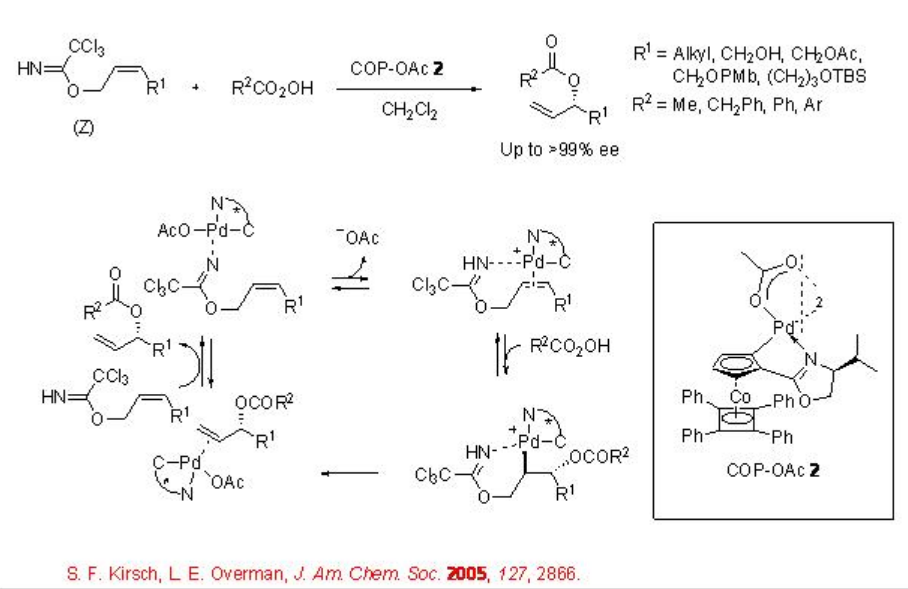
This procedure has also been extended for the allylic etherification reaction. For example, the reaction of ( Z )-allylic trichloroacetimidates with carboxylic acids in the presence of COP-OAc 2 gives chiral allylic esters in high enantiopurity (Scheme \(\PageIndex{3}\)). Under these reaction conditions, E -stereoisomer show inferior results. In these reactions, the COP-OAc activates the carbon-carbon double bond for attack by external oxygen nucleophile and the trichloroacetimidate group serves as a leaving group along with templating the catalyst to the double bond.