3.1: Reactions with Metal Carbenoid
- Page ID
- 168780
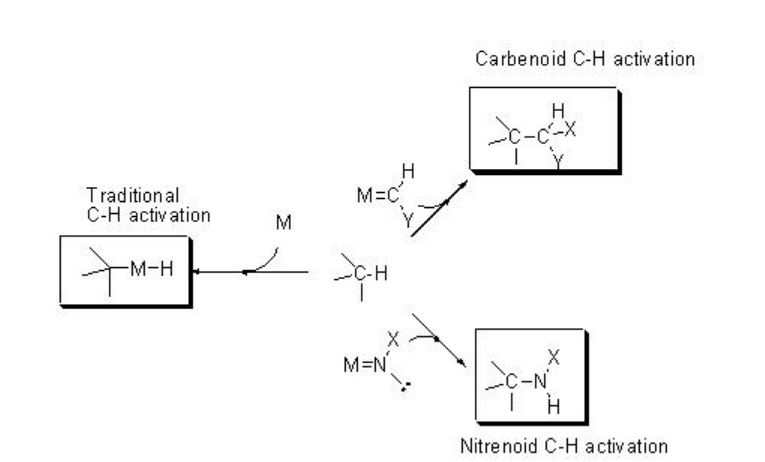
Functionalization of C-H bonds constitutes an attractive approach for the direct synthesis of complex organic molecules such as pharmaceuticals, natural products, and other industrially relevant targets. Thus, much effort has been devoted to achieve practical, catalytic and selective methods for the C-H functionalization. Scheme \(\PageIndex{1}\) presents the two major directions evolved for the C-H functionalization process: (i) direct C-H activation involving oxidative addition to the C-H bond onto an active metal center, and (ii) insertion of transition metal-coordinated carbenes or nitrenes into the C-H bond to give functionalized products.
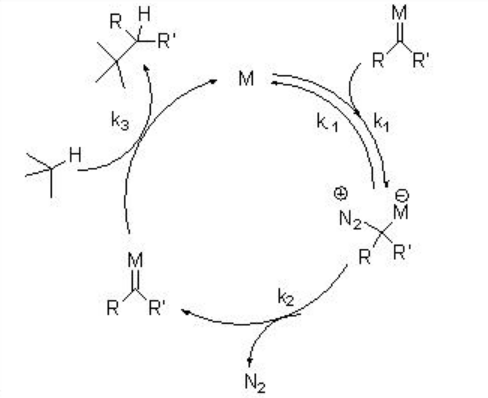
Metal carbenes generally produced from diazo compound by metal-catalyzed nitrogen extrusion. Alternative carbene precursors include iodonium, sulfonium, sulfoxonium, thiophenium and phosphonium ylides, but their synthetic application is less explored. The general mechanism for the generation of carbene via dirhodium complexes is shown in Scheme \(\PageIndex{2}\). In the presence of suitable metal complex, the diazo compound can coordinate reversibly and undergo rate limiting extrusion of nitrogen to give reactive metal carbenoid intermediate. The latter will react with a suitable trapping agent present in the reaction mixture.
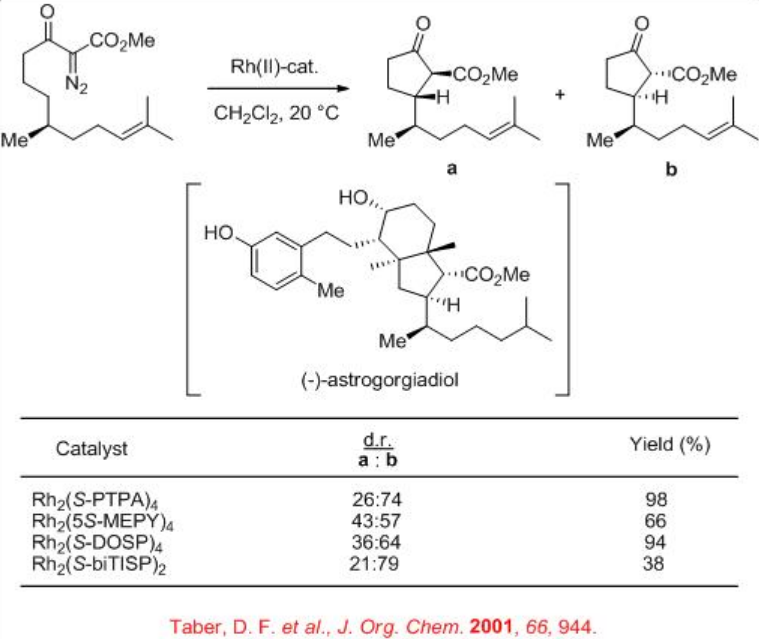
For example, chiral dirhodium complexes catalyze the intramolecular C-H insertion of α -diazo -β -ketoester to give the intermediate for the total synthesis of the marine secosteroid (-)-astrogorgiadiol (Scheme \(\PageIndex{3}\)). Up to 58% de is observed with moderate yield of 38% employing Rh2( S -biTISP)2 as the catalyst. The reaction using Rh2 (S-PTPA)2 afforded excellent yield but with lower diastereoselectivity.
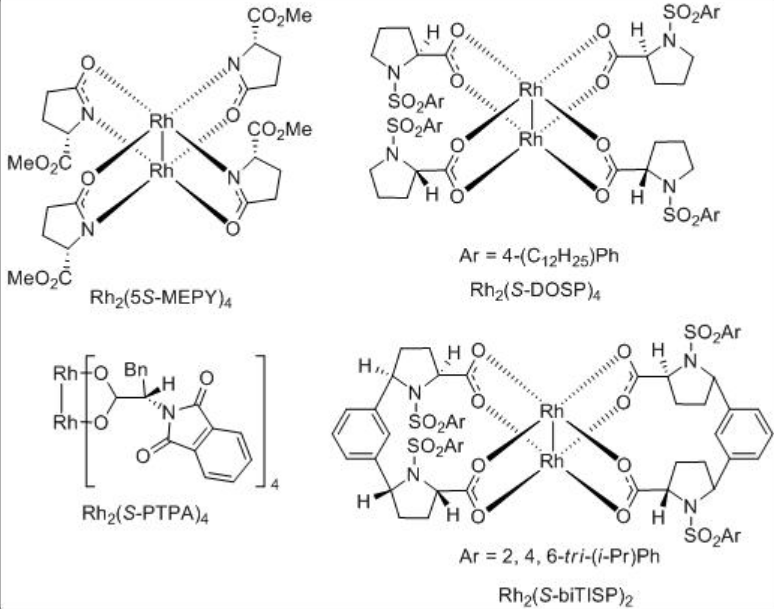
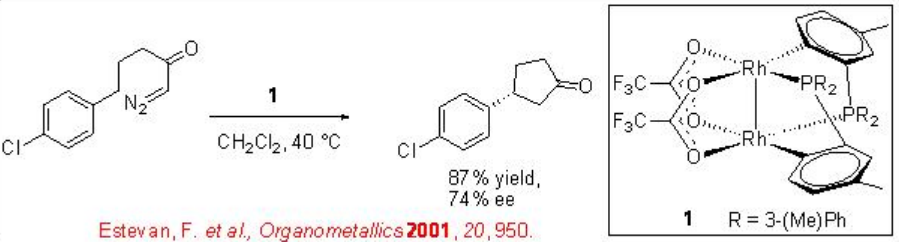
ortho -Metallated arylphosphine dirhodium(II) complexes are found to be effective catalysts for intramolecular C-H insertions of certain diazoketones (Scheme \(\PageIndex{4}\)). One of the examples is the use of dirhodium complex 1 for the reaction of chloro-substituted system to afford cyclophentanone in 74% ee and 87% yield. This system works well with the aryl portion having electron withdrawing group.
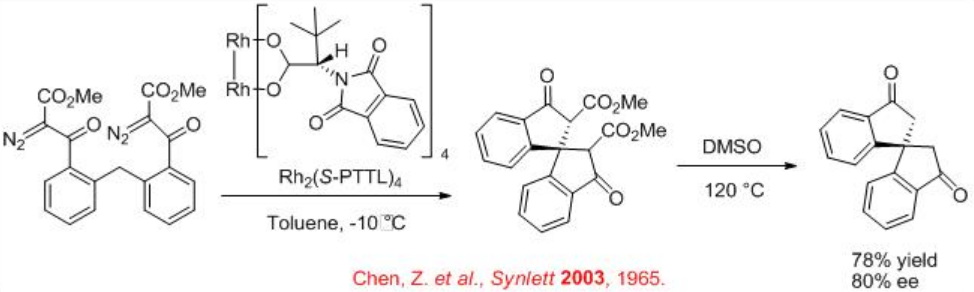
Scheme \(\PageIndex{5}\) illustrates an example for the stereocontrolled formation of quaternary stereocenter using chiral Rh2 (S-PTTL)4 catalyzed carbenoid C-H insertion process.
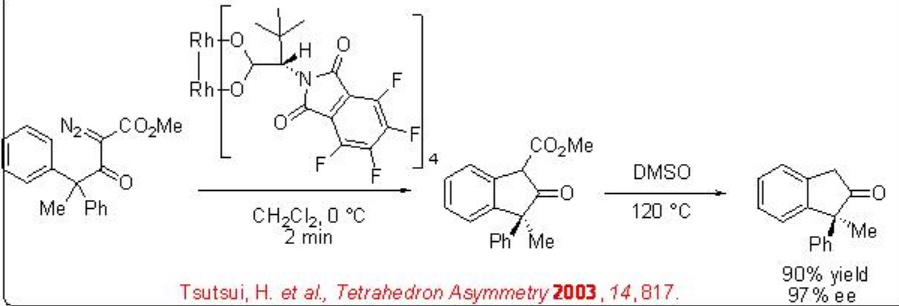
The above catalytic system is also effective for the desymmetrization of aryl-substituted diazo ketoesters (Scheme \(\PageIndex{6}\)). This reaction proceeds via electrophilic aromatic substitution and turnover numbers of up to 98000 have been achieved.
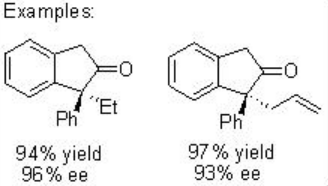
Furthermore, the construction of cis -cyclopentanones from diazoester can be achieved via exclusive insertion (Scheme \(\PageIndex{7}\)). In addition, the construction of disubstituted cis -indane can be accomplished with 85% yield and 92% ee (Scheme \(\PageIndex{8}\)). These examples illustrate that the choice of the reaction conditions and catalysts for carbenoid transformation are crucial for selectivity.
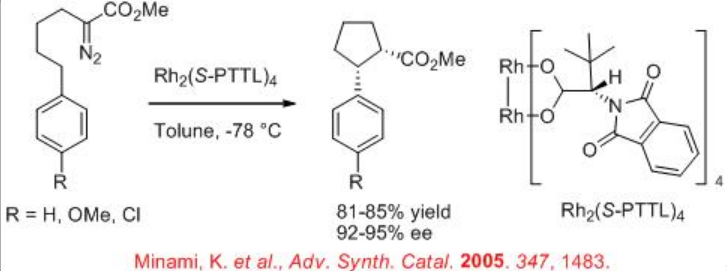
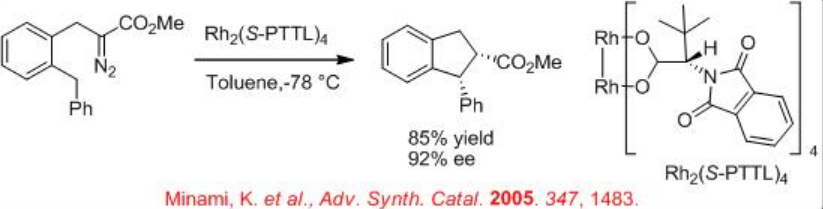
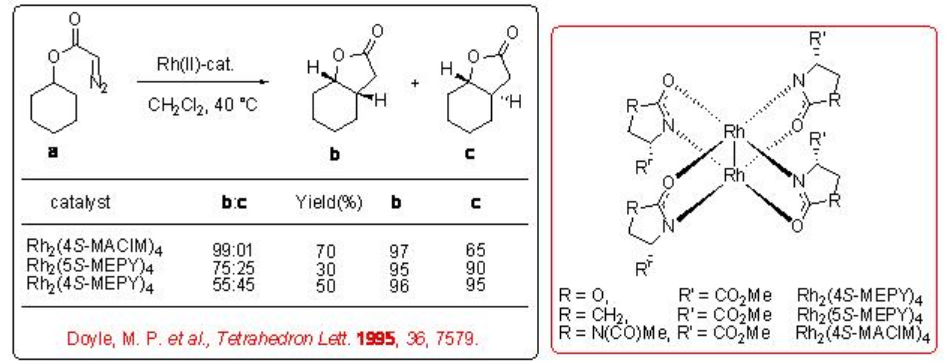
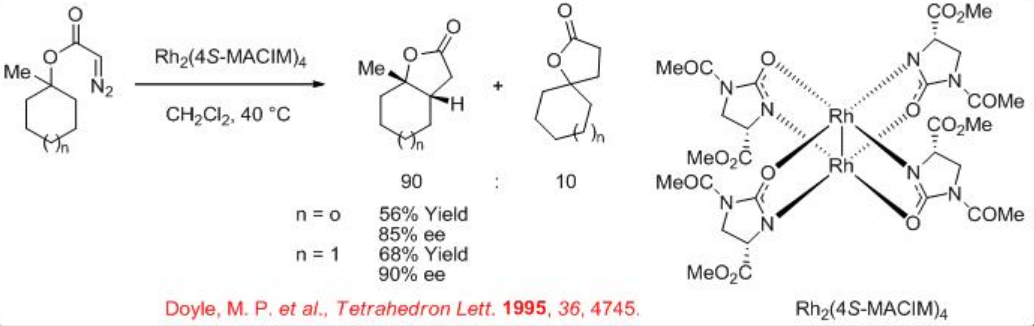
Both the first (Rh2(MEOX)4 and Rh2 (MEPY)4) and second (Rh2(4S-MACIM)4) generation carboxamidate catalysts show very good enantiocontrol for the desymmetrization reaction of cyclohexyl diazoacetate (Scheme \(\PageIndex{9}\)). In terms diastereoselectivity, the latter gives the best results of 99:1 which is attributed to the N -substituent that control the carbenoid orientation.
In case of cyclohexyl diazoacetate having the tertiary system, a mixture of the expected insertion into methylene group and insertion into the methyl group has been observed in the presence of Rh2(4S-MACIM)4 (Scheme \(\PageIndex{10}\)). Cyclopentane system also provides similar results with somewhat lower yield and enantioselectivity.
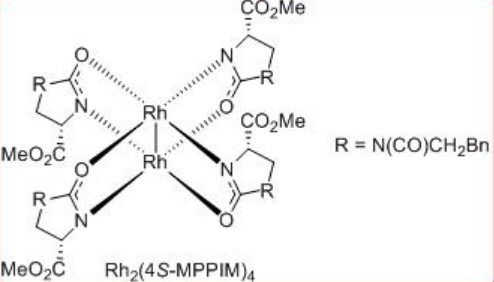

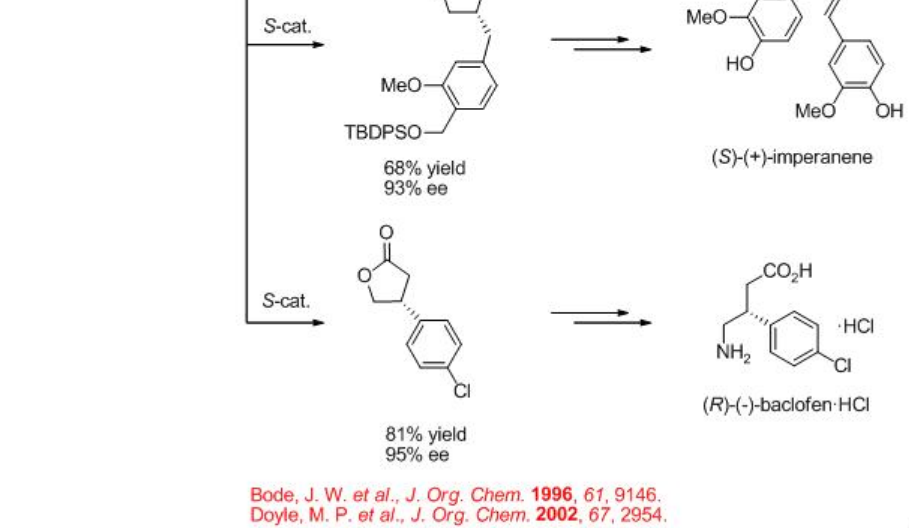
The construction of γ -lactone has been demonstrated via intramolecular C-H insertion of diazoacetates that find wide applications in the synthesis of natural products and pharmaceutical agents. For example, the synthesis of (+)-isodeoxypodophyllotoxin, (-)-enterolactone, (S)-(+)-imperanene and (R)-(-)-baclofen have been accomplished with the lactone formation as a key step in the presence of Rh2 (4S/R-MPPIM)4 (Scheme \(\PageIndex{11}\)).
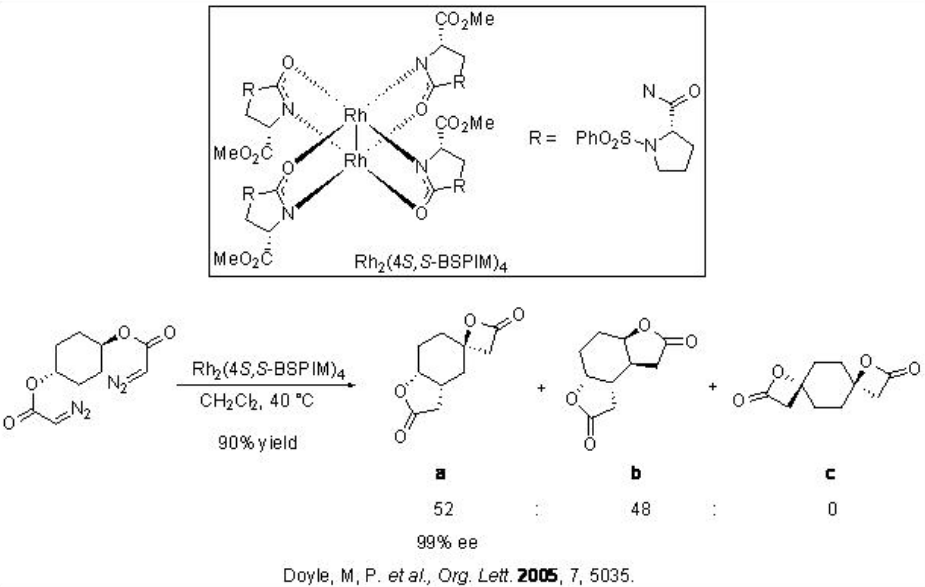
The carbenoid insertion reactions have also been used for amplification of asymmetric induction. For example, sequential intramolecular C-H insertions have been carried out on meso -cyclohexyl diazoacetate (Scheme \(\PageIndex{12}\)). The formation of a 1:1 mixture of a and b is observed using Rh2(4 S,S -BSPIM)4 with over 90% yield and 99% enantioselectivity.
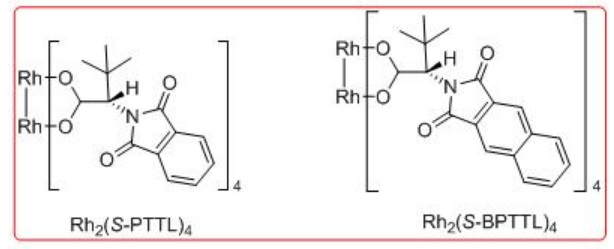
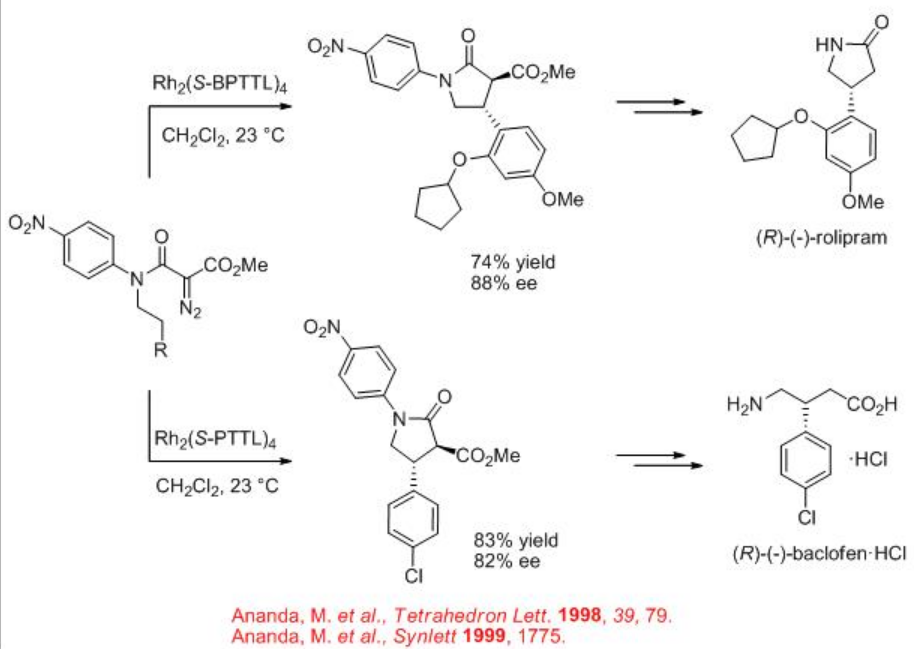
Synthetic application of the dirhodium catalyzed carbenoid C-H insertion chemistry has been demonstrated as key step for the site controlled γ -lactam formation to the syntheses of (R)-(-)-baclofen, GABAB receptor agonist and (R)-(-)-rolipram (Scheme \(\PageIndex{13}\)). Rh2 (S-BPTTL) is found to be the optimal catalyst for the synthesis of the intermediate for (R)-(-)-rolipram with 74% yield and 88% ee, while Rh2 (S-BPTTL) is effective for the synthesis of the intermediate to (R)-(-)-baclofen with 83% yield and 82% ee.
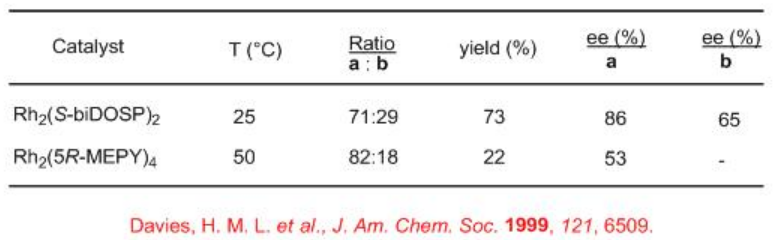
So far we have seen intramolecular carbenoid C-H insertion reactions. Intermolecular carbenoid C-H insertion reactions have been recently explored. Scheme 6 illustrates the reaction N-Boc piperidine with methylphenyldiazoacetate in the presence of Rh2(S-biDOSP)2 at ambient temperature. Two diastereomers in a 71:29 ratio is formed in overall 73% yield and up to 86% ee. The racemic threo -methylphenidate is currently marketed drug for treatment of attention hyperactivity disorder. Seven and eight member nitrogen heterocycles afford higher selectivity. The use of dirhodium carboxamidate Rh2(5R-MEPY)4 for this chemistry shows improved diastereoselectivity but with low yield and enantioselectivity.