2.4: Metal-Catalyzed Asymmetric Conjugate Addition Reactions
- Page ID
- 168776
Asymmetric conjugate addition is one of the powerful tools for the construction carbon-carbon and carbon-heteroatom bonds in organic synthesis. This reaction finds extensive applications for the construction enantioenriched carbon skeletons for the total synthesis of numerous biologically active compounds. Sometimes possible to construct multiple stereocentres in single synthetic operation. This lecture covers some examples for the recent developments in the conjugate addition of Grignard, organozinc, organolithium, organocopper and organoborane reagents with activated alkenes in the presence of chiral ligand or chiral catalysts.
Reactions of Grignard Reagents
The conjugated addition of Grignard reagents with electrophilically activated alkenes is well explored. Some of the chiral ligands developed for the conjugate addition reactions of Grignard reagents with α,β -unsaturated carbonyl compounds are shown in Scheme \(\PageIndex{1}\).
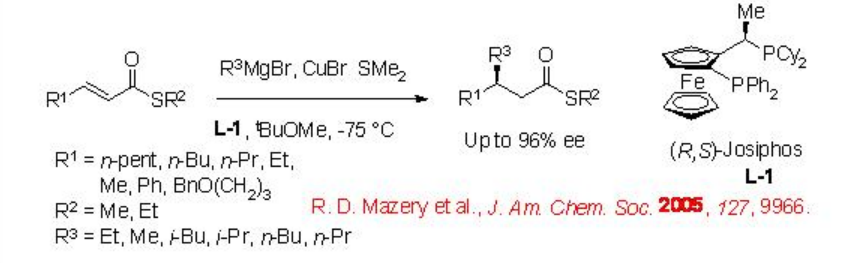
One of the recent examples is the addition of alkyl magnesium bromide to α,β -unsaturated thioesters using Josiphos ligand L-1 (Scheme \(\PageIndex{2}\)). The reactions of a series of examples can be accomplished with up to 96% enantioselectivity.
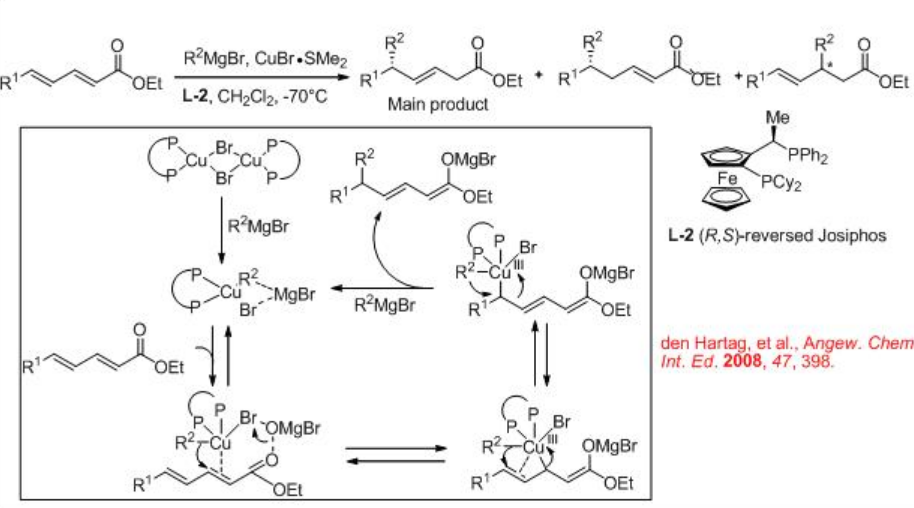
Compared to the 1,4-conjugate addition reaction, the reactions with extended Michael acceptors needs additional control of the regioselectivity. For example, using the ( R,S )-reversed Josiphos ligand L-2 , 1,6-asymmetric conjugate addition to α,β,γ,δ -unsaturated esters has been developed (Scheme \(\PageIndex{3}\)).
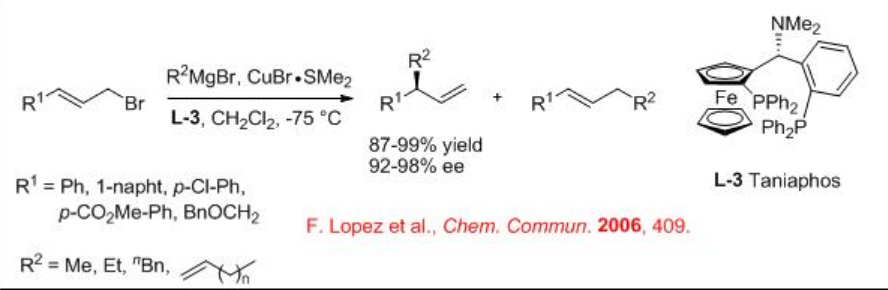
Besides the ferrocenyl ligands L1-2 , taniaphos L-3 with CuBr∙SMe2 is also highly effective for the conjugate addition of allylic electrophiles with Grignard reagents (Scheme \(\PageIndex{4}\)). In this reaction, aliphatic allylic bromides have been found to be excellent substrates.
Reactions of Organozinc Reagents
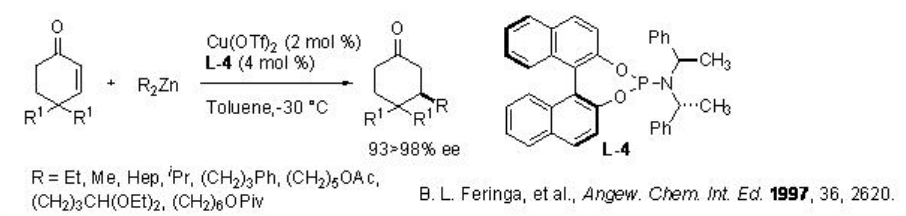
The asymmetric conjugate addition of dialkylzinc to prochiral α,β -unsaturated compounds is one of the powerful methods for carbon-carbon bond formation in organic synthesis. Much attention has been made on the development of new ligands for this reaction. Phosphoramidite ligand from BINOL L-4 has been found to be effective for the conjugate addition to cyclic substrates with up to 98% ee (Scheme \(\PageIndex{5}\)).
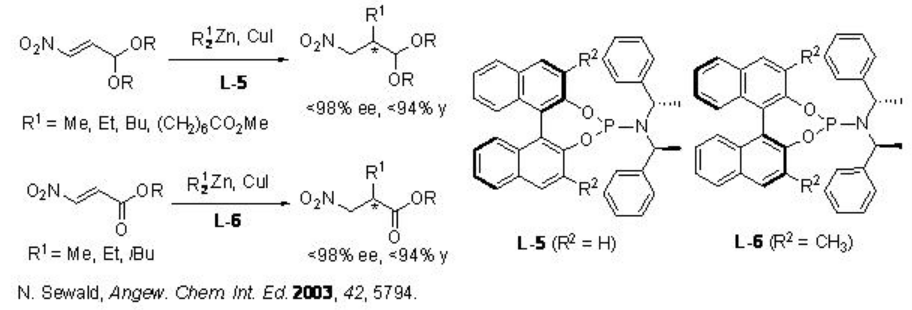
Subsequently, copper(I)-catalyzed enantioselective addition of dialkylzinc to 3-nitroacrolein derivatives has been demonstrated using phosphoramidite ligands L-5 and L-6 with up to 98% ee (Scheme \(\PageIndex{6}\)).
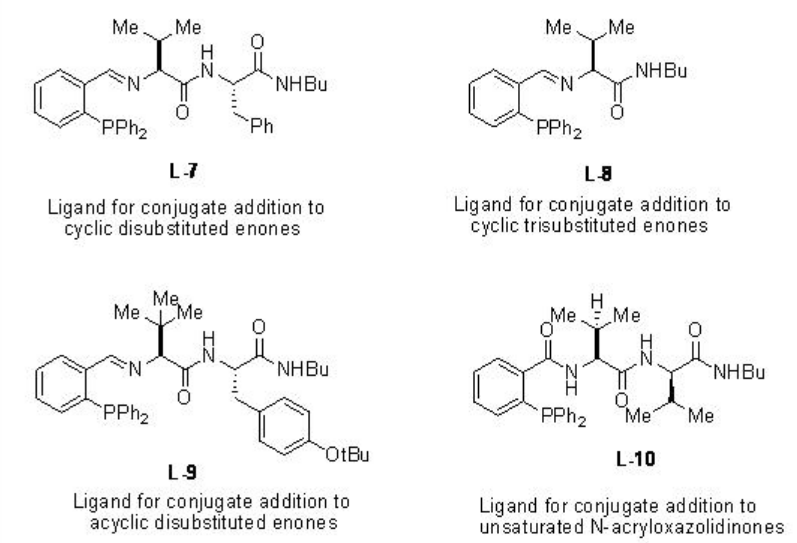
Scheme \(\PageIndex{7}\) summarizes some of the peptide based ligands for the dialkylzinc addition to α,β -unsaturated compounds. For example, the copper-catalyzed conjugate addition of dialkylzinc reagents to acyclic aliphatic α,β -unsaturated ketones proceed in the presence of L-9 with up to 94% ee, while the reaction using L-10 gives up to 98% ee (Scheme \(\PageIndex{8}\)).
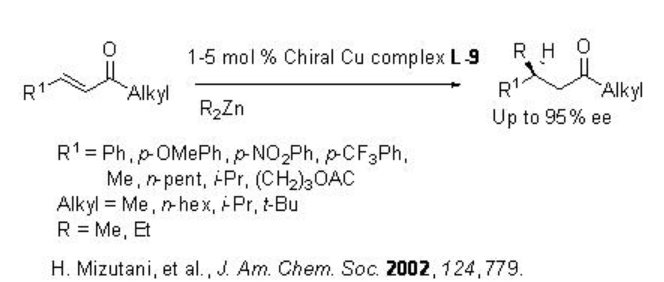
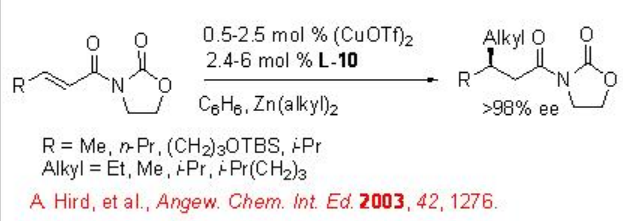
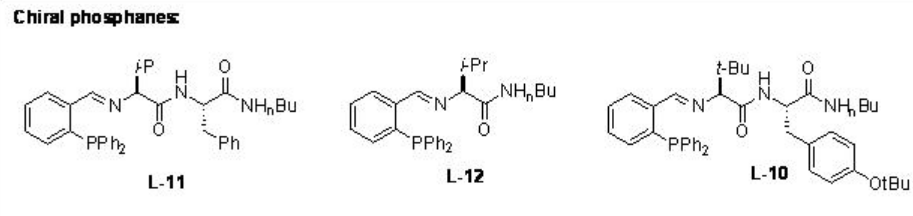
Later, the chiral ligands L-10 to L-12 have been studied for the reactions of dialkylzinic reagents to heterocyclic enones such as furanones, pyranones and their derivatives (Scheme \(\PageIndex{9}\)-\(\PageIndex{10}\)).
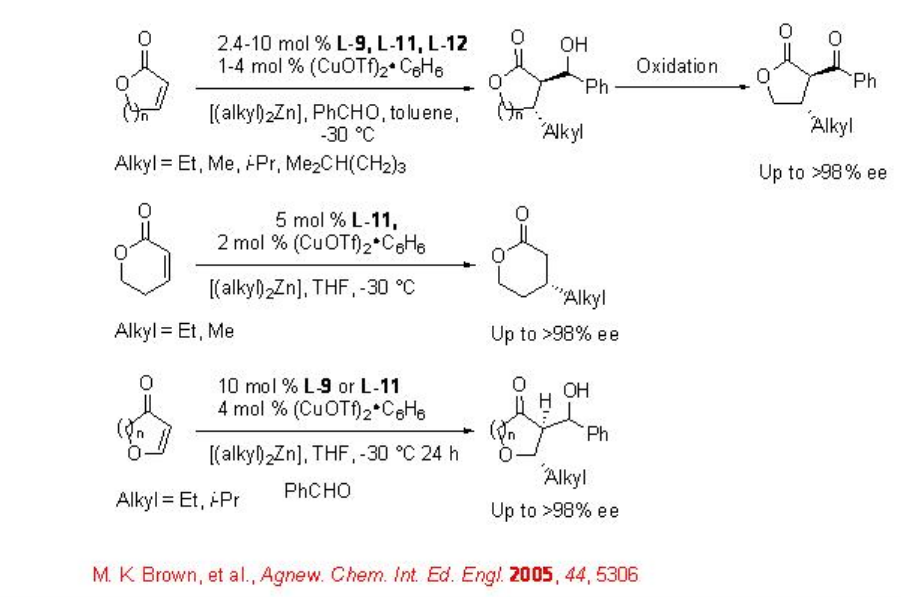
Reactions of Organolithium Reagents
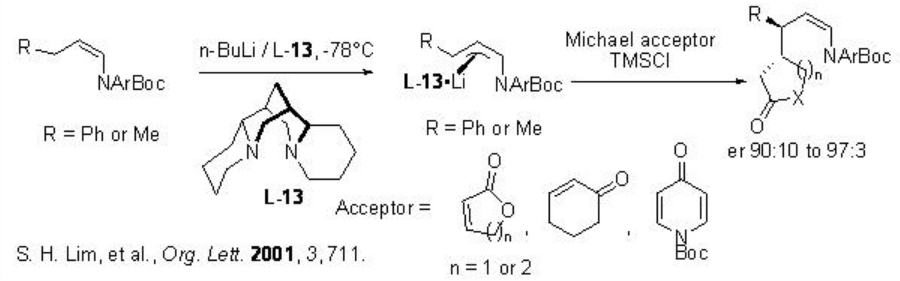
Organolithium reagents are highly reactive species and their conjugate addition reactions with α,β-unsaturated carbonyl compounds are of great interests. One of the recent examples is the reaction of configurationally stable organolithium to α,β -unsaturated cyclic carbonyl compounds using (-)-sparteine that can be performed with high enantioselectivity (Scheme \(\PageIndex{11}\)).
Reactions of Organoboranes
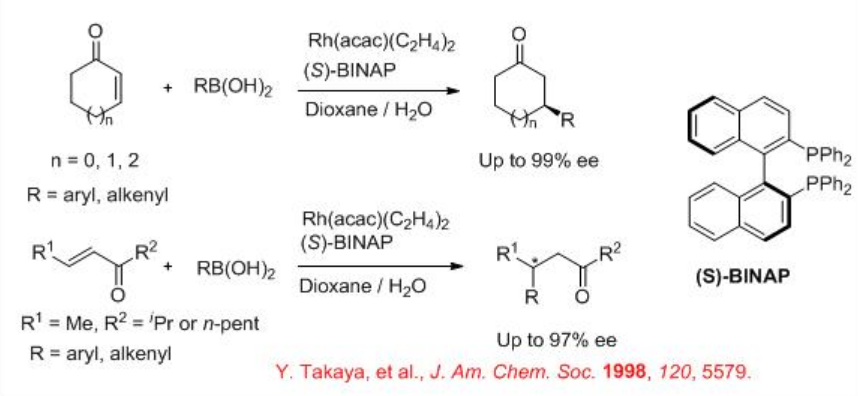
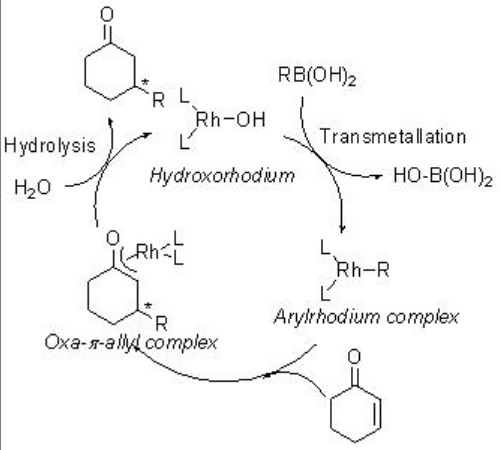
The asymmetric conjugate addition of organoboranes using chiral rhodium phosphine complex is a successful process. For example, arylboronic and alkenylboronic acids undergo reaction with cyclic and acyclic α,β -unsaturated ketones in the presence of chiral rhodium complex bearing (S)-BINAP with high enantioselectivity (Scheme \(\PageIndex{12}\)). The reaction proceeds via phenylrhodium, oxa- π -allylrhodium and hydroxorhodium intermediates (Scheme \(\PageIndex{13}\)).
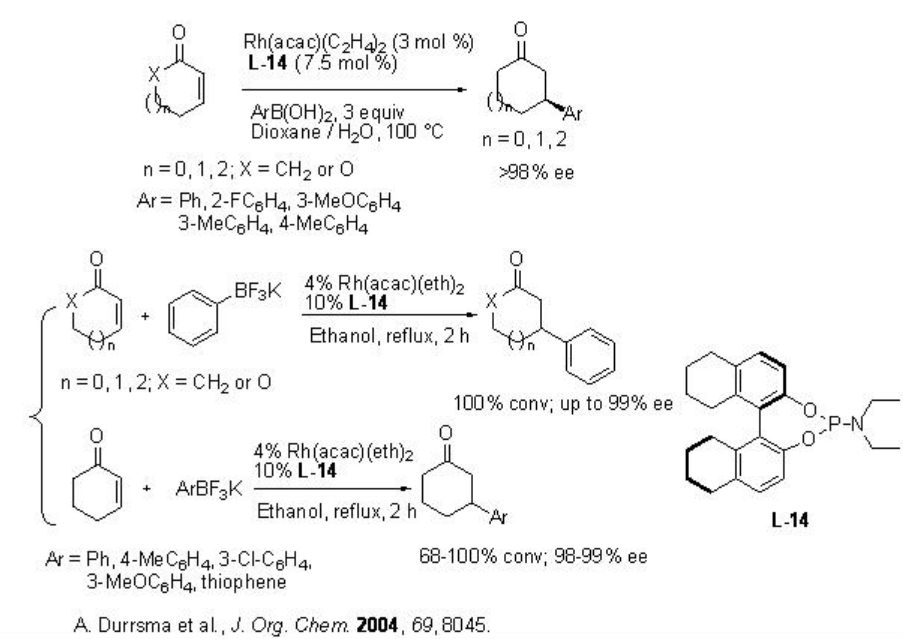
Besides chiral biphosphines, chiral dienes and chiral phosphoramidite ligands are also effective for the rhodium catalyzed conjugate addition of organoboranes. For example, the rhodium catalyzed conjugate addition of boronic acids and potassium trifluoroborates to enones occurs with high enantioselectivity (Scheme \(\PageIndex{14}\)).