2.2: Enantioselective Alkene Metathesis
- Page ID
- 168774
Among the alkene metathesis catalysts, Mo and Ru-based complexes have emerged as powerful exhibiting complementary reactivity and functional group tolerance. The asymmetric alkene metathesis provides access to enantiomerically enriched molecules that can not be generally prepared through the commonly practiced strategy. Unlike most of the other enantioselective processes, alkene metathesis, which entails the formation and cleavage of carbon-carbon double bonds, does not involve the direct construction of sp 3 -hybridized stereogenic center. Instead, the stereochemistry is created indirectly, often by desymmetrization of an achiral substrate (Scheme \(\PageIndex{1}\)), wherein the chiral catalyst has to discriminate between enaniotopic groups or sites of the molecule.
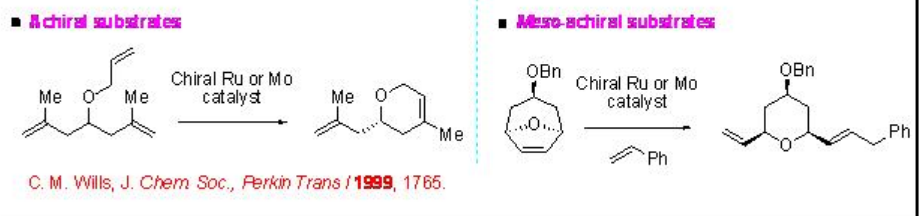
Scheme \(\PageIndex{1}\): Desymmetrization in catalytic enantioselective alkene metathesis .
Ring-Closing Metathesis (RCM) Reactions
2.2.1.1 Ru-Catalyzed Reactions
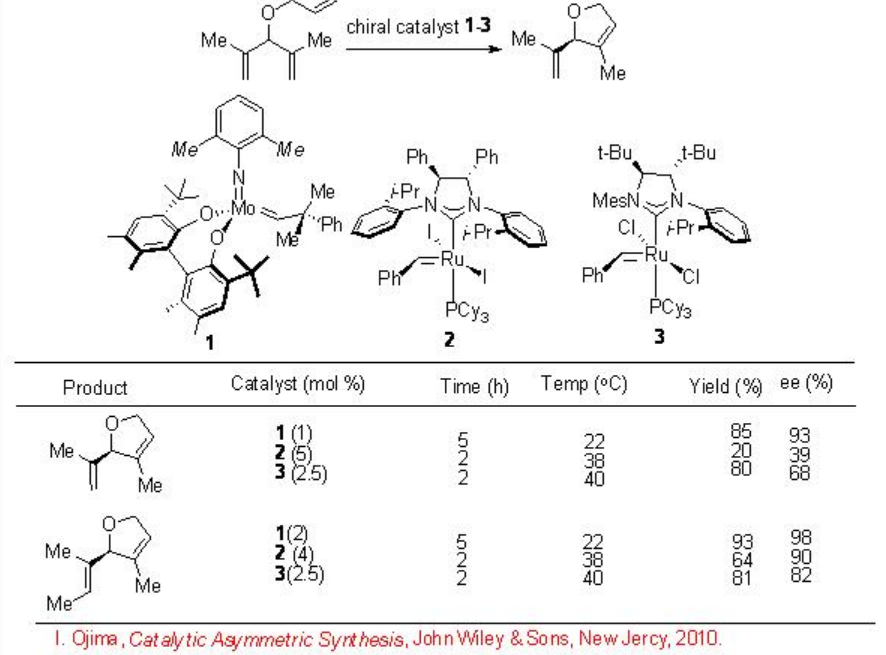
RCM is most commonly used in organic synthesis to construct cyclic systems, which are sometimes difficult to prepare by most of the other methods. During the past decade, several Ru and Mo-based chiral catalysts have been developed for the enantioselective RCM process and made remarkable progress. Scheme \(\PageIndex{2}\) summarizes examples for enantioselective RCM employing monodentate chiral NHC-Ru and chiral Mo-diolate complexes. The Ru-based catalysts are selective compared to Mo-based one, which catalyzes a wide range of substrates.
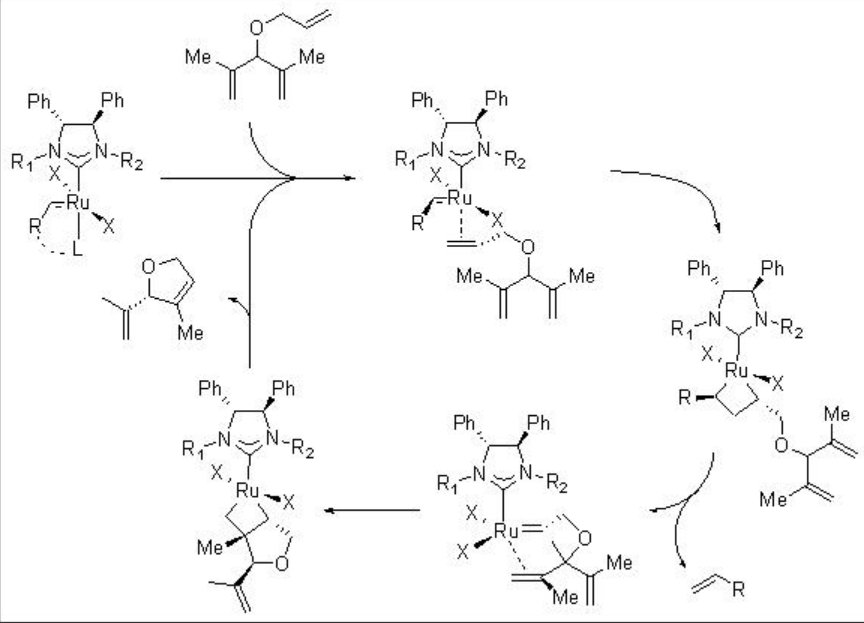
The mechanism of the Ru-catalyzed RCM is outlined in Scheme \(\PageIndex{3}\). Initiation of the reaction may take place via the dissociation of either the phosphine ligand or chelated etherate moiety. Subsequently, the less substituted alkene may make coordination to the Ru center, which could proceed [2+2]-cycloaddition, followed by cycloreversion and ruthenacyclobutane formation that could lead to the target product. The formation and cleavage of the cyclobutanes are crucial for the enantioselectivity of the products.
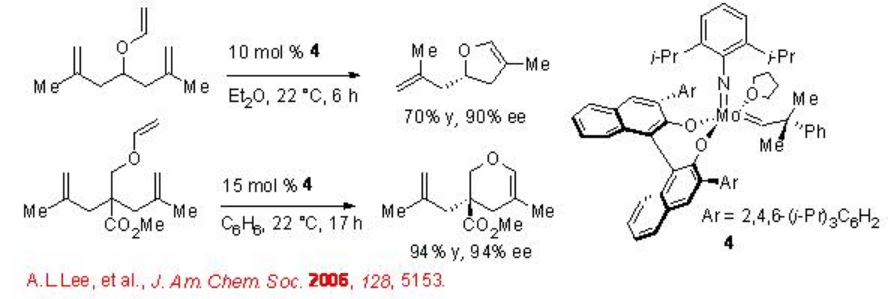
2.2.1.2 The Synthesis of Cyclic Enol Ethers using Mo-Catalyzed RCM
Mo-based RCM is found to be successful for the synthesis of furan and pyran products with up to 98% ee (Scheme \(\PageIndex{4}\)). Although high catalyst loading is required, the products can be constructed with tertiary and quaternary stereogenic centers. In contrast, the Ru-based catalysts are not successful for this transformation.
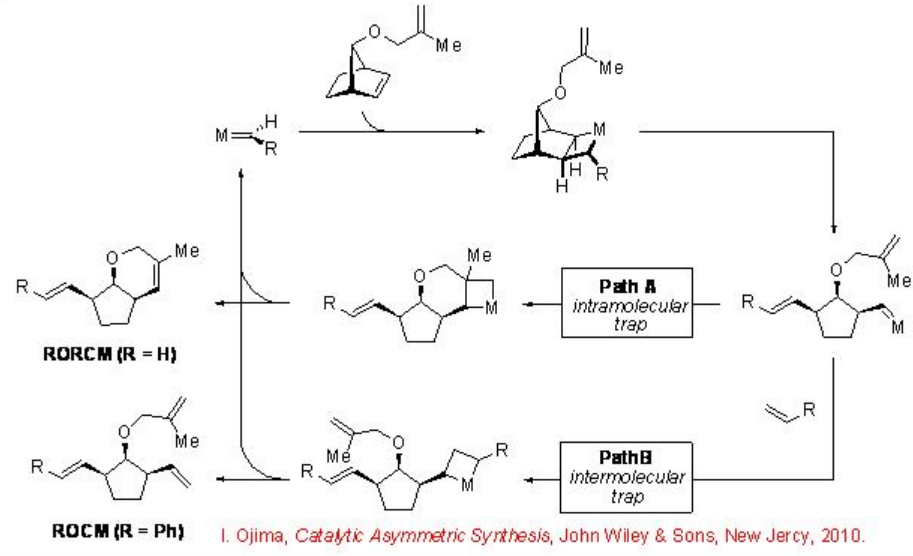
Ring-Opening/Ring-Closing Metathesis (RORCM) and Ring-Opening/Cross Metathesis (ROCM)
Following the ring opening, the resulting carbene intermediate can be traped intramolecularly by a pendant alkene (RORCM, Path A) or intermolecularly using a cross-partner (ROCM, Path B) (Scheme \(\PageIndex{5}\)). These reaction pathways can be controlled by selection of the appropriate catalyst and cross partner, which can lead to a wide range of enantiomerically enriched products from common starting material. In the absence of intramolecular trap (ROCM process), a number of complex mixture of products can be generated.
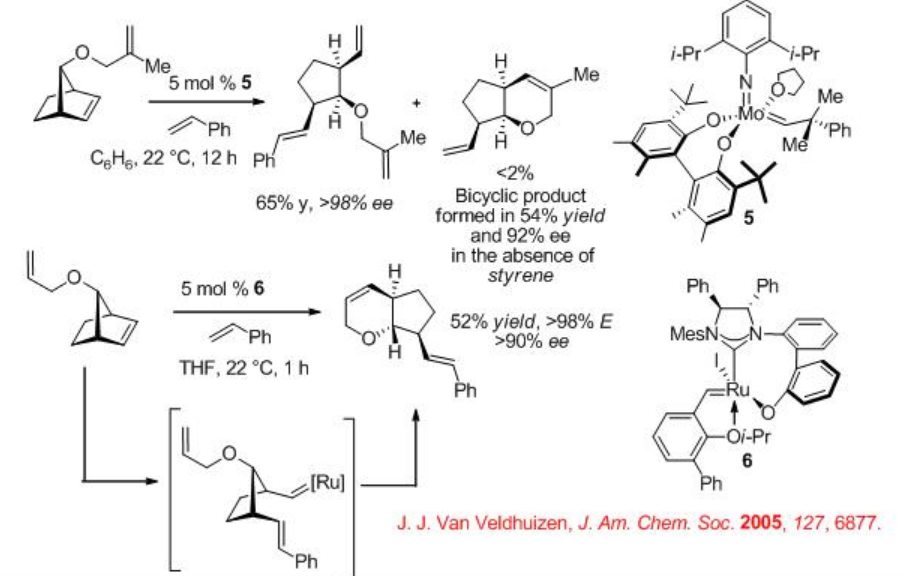
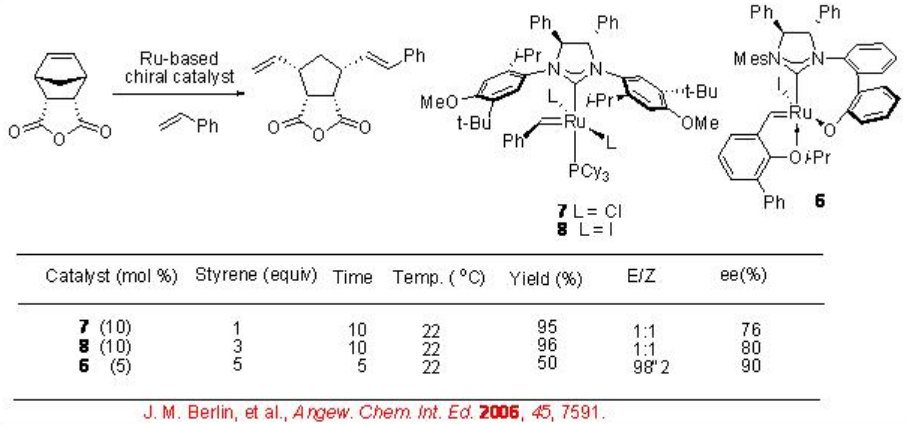
Scheme \(\PageIndex{6}\) presents examples for the Mo and Ru-catalyzed enantioselective ROCM processes. Norbornenes react with styrene via ROCM with high enantioselectivities. In both cases, E -alkenes are generated. In the absence of styrene, in the case of Mo-based system, RORCM product is formed with 92% ee. The substrate used for the Ru-catalyzed ROCM process, proceed polymerization in the presence of Mo-catalyst instead of ROCM process.
Scheme \(\PageIndex{7}\) shows the comparison of the Ru-catalyzed ROCM of norbornenes. The catalysts 7 and 8 bearing monodendate NHC ligands exhibit greater reactivity (i.e., lower catalyst loading) compared to the complex bearing bidendate NHC ligand 6 . But the systems using 7 and 8 produce poor E / Zselectivity, whereas the reaction using 6 gives exclusively E -isomer.
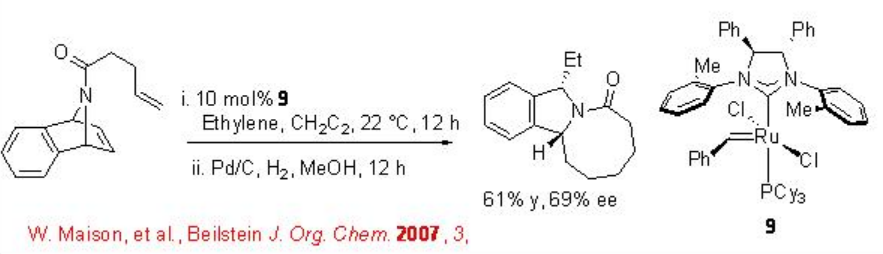
The synthesis of isoindole has been recently shown using chiral Ru-catalyzed RORCM with moderate enantioselectivity (Scheme \(\PageIndex{8}\)). In this reaction the use of ethylene is to facilitate the release of the catalyst. The direct alkene metathesis product is unstable and thus it was isolated after hydrogenation.
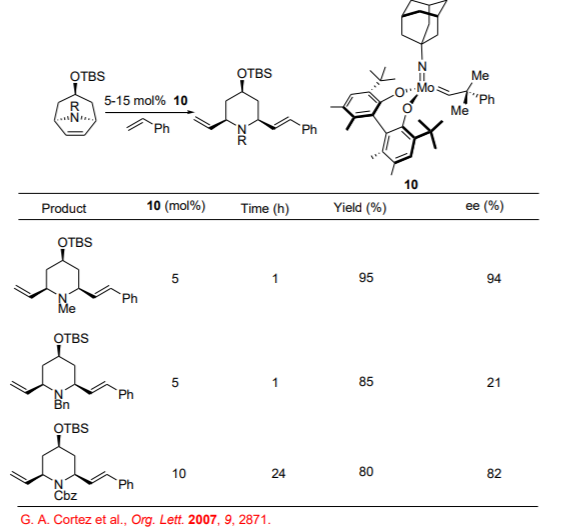
2,6-Disubstituted piperidines are important structural unit present in medicinally significant compounds. Using the Mo-based enantioselective ROCM reactions, the synthesis of the N -protected 2,6-substituted piperidines can be accomplished from of meso -azabicycles with moderate to high enantioselectivities (Scheme \(\PageIndex{9}\)).
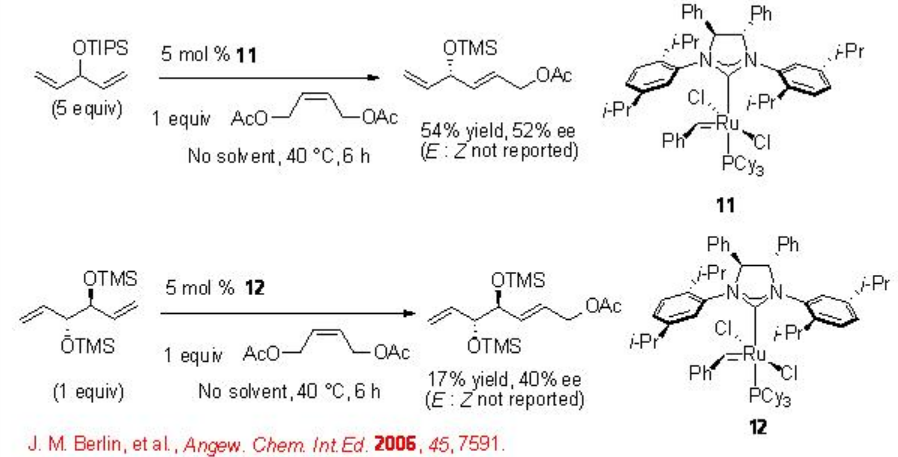
Cross-Metathesis (CM)
Catalytic enantioselective CM is least developed in enantioselective alkene metathesis reactions. Unlike the ring-closing and ring-opening metatheses that are thermodynamically driven, there is minimal driving force for the CM. In addition, selectivity between two different cross partners leads to complex. Scheme \(\PageIndex{10}\) presents some examples of CM using chiral Ru complexes with moderate enantioselectivity. These substrates don't proceed RCM due to ring strain of the products.