11.1: Acylation of Alcohols and Amines
- Page ID
- 168836
Biocatalysis is a highly efficient and a powerful tool for organic chemists to prepare optically pure molecules. A broad range of biocatalytic methods has been already in use for large-scale manufacture of drug intermediates. This module covers some of the recent developments in the enzyme catalysis.
The enzymatic resolution of alcohols and amines affords an effective method to access optically active alcohols and amines from racemic or prochiral substrates.
Reactions with Alcohols
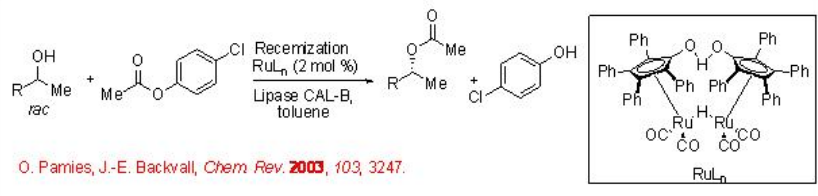
The use of lipase for the resolution of racemic alcohols is a widely known technology. However, this method gives the product with maximum up to 50% yield. This limitation can be overcome by coupling the lipase-catalyzed enantioselective resolution with a racemization of the alcohol substrate, thus obtaining a dynamic kinetic resolution process. The latter process can be pursued employing a nonchiral metal complex as a catalyst. For example, using the combination of Ru complex and CAL-B, the acylation of racemic alcohol can be accomplished with 78-92% yield and 99% ee (Scheme \(\PageIndex{1}\)).
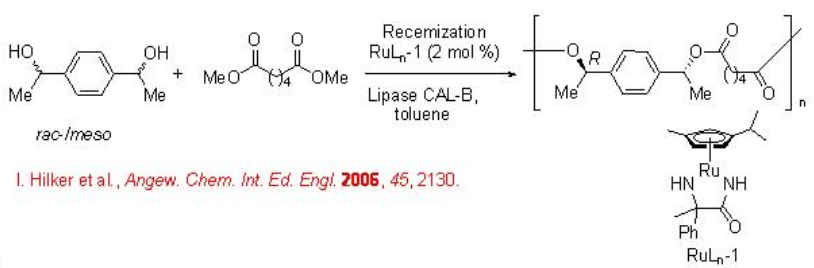
This methodology has been subsequently utilized for the enantio- and diastereoselective synthesis of chiral polymers. For example, dimethyl adipate reacts with a mixture of racemic and meso -alcohols to give chiral polyester (Scheme \(\PageIndex{2}\)). Ru complex acts as a racemization catalyst in combination with lipase CAL-B as biocatalyst for the resolution.
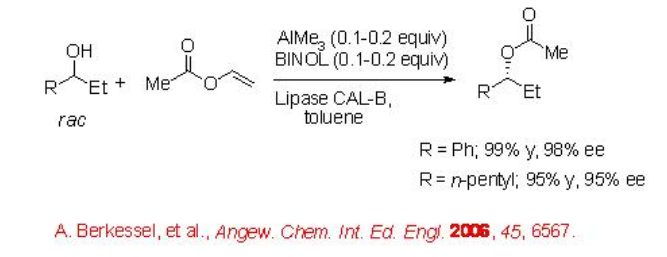
Furthermore, the transformation has been demonstrated employing a cheap and readily available aluminium complex prepared from AlMe3 and BINOL as the racemization catalyst. For example, racemic 1-phenyl-1-propanol can be acylated with 99% yield and 98% ee (Scheme \(\PageIndex{3}\)).
11.1.2 Reactions with Amines
Optically pure amines serve as versatile intermediates in the manufacture of pharmaceuticals and agrochemicals. The lipase-catalyzed acylation of amines proceeds efficiently with excellent enantioselectivity (Scheme \(\PageIndex{4}\)). In this reaction, one of the enantiomer is converted into amide and the remaining amine enantiomer can be obtained in enantiomerically enriched form. The reaction functions in organic medium, MTBE as solvent, and E value exceeds 2000 (E = environmentally impact of the process.
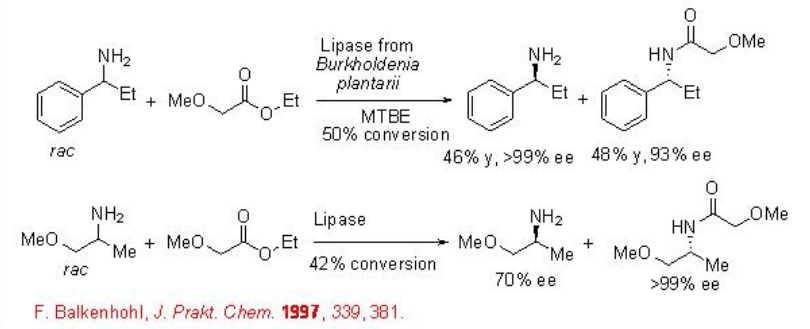
11.1.3 Other Acylations
Enzymatic catalytic transformation of achiral amines and racemic acid components known as aminolysis affords elegant approach for the synthesis of enantioenriched acids. An interesting example is the reaction of dimethyl 3-(benzylamino)glutarate to give monoamides with excellent enantioselectivity (Scheme \(\PageIndex{5}\)). The monoamides are intermediates for the synthesis of unnatural β -amino acids.
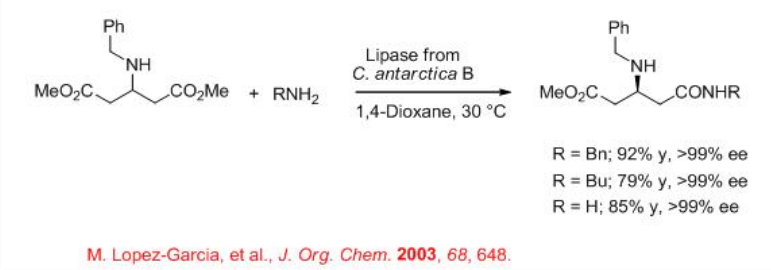
A dynamic kinetic resolution with enzymatic aminolysis provides effective route towards the access of enantiomerically enriched acids. For example, in the presence of an immobilized phosphonium chloride for racemization of ethyl 2-chloropropionate and lipase, aminolysis can be carried out to give amides with up to 92% yield and 86% ee (Scheme \(\PageIndex{6}\)).
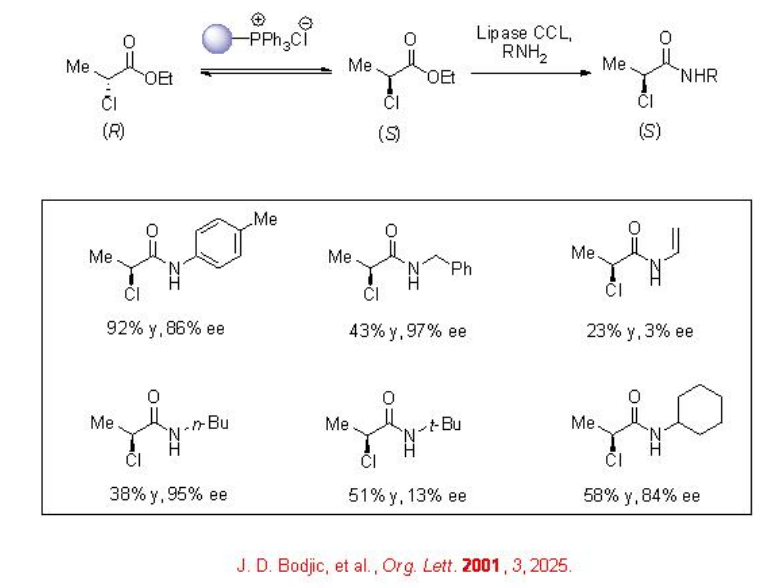
Hydrolytic Reactions
The enzymatic hydrolysis of racemic esters, amides, nitriles and epoxides affords effective methods for the synthesis of optically pure carboxylic acids, amines, amides, esters and alcohols. The reactions of a broad range of substrates have been well explored.
Ester Hydrolysis
Hydrolysis of racemic or prochiral ester using enzymes such as lipase, esterase and protease provides effective method for the resolution of broad range of substrates. Recently, the hydrolysis of indole ethyl ester has been shown using a lipase from Pseudomonas fluoresens (Scheme \(\PageIndex{7}\)). The process runs at a high substrate concentrate 100g/L and turned out to be technically feasible to perform successfully on a 40-kg scale.
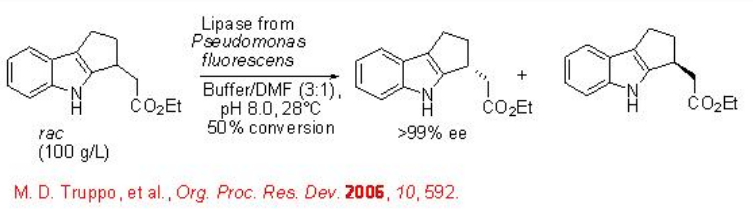
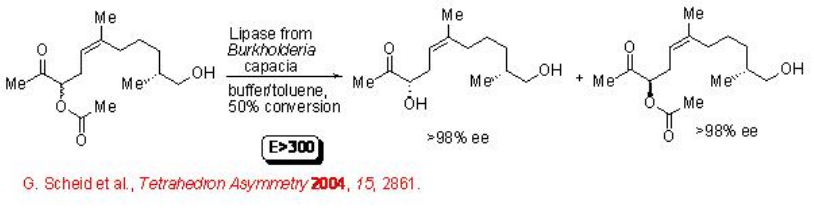
Lipases are also suitable for the resolution of complex molecules having more than one additional functional group. For example, acyloin acetate can be hydrolyzed with E > 300 leading to diol in excellent enantioselectivity (Scheme \(\PageIndex{8}\)).
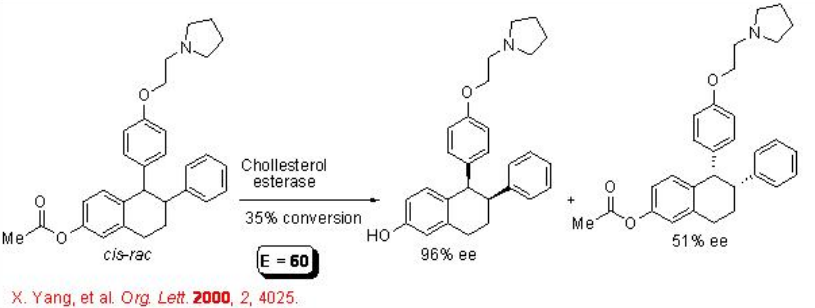
Hydrolases can also recognize “remote chiral centers”. For example, ester group separated from the stereogenic center by an aromatic group proceeds hydrolysis with enantioselectivity having the E value of 60 (Scheme \(\PageIndex{9}\)). The product, Lasofoxifene ( cis ), is a potent and selective estrogen receptor modulator.
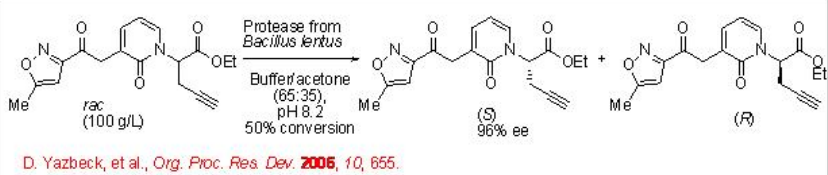
The synthesis of an intermediate for a rhinovirus protease inhibitor has been accomplished by an impressive resolution employing a protease from Bacillus lentus (Scheme \(\PageIndex{10}\)).
Nitrile Hydrolysis
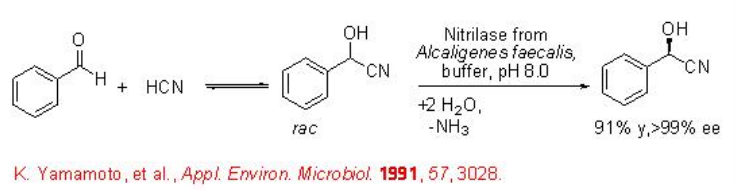
Nitrilases are used for the hydrolysis of racemic or prochiral nitriles to give carboxylic acids. For example, nitrilase from A. faecalis catalyzes the hydrolysis of α -hydroxy nitriles to give ( R )-mandelic acid with excellent enantioselectivity (Scheme \(\PageIndex{11}\)).
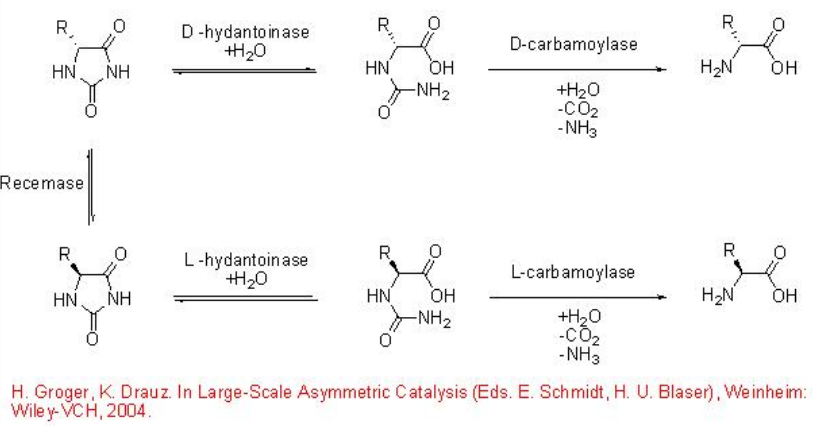
11.2.3 Hydantoin Hydrolysis
Hydantoinases and carbamoylases hydrolyses racemic hydantoins to give optically pure α -amino acids (Scheme \(\PageIndex{12}\)). In the beginning, the hydantoinase catalyzes the hydrolytic ring opening of the hydantoin to give an N -carbamoyl amino acid that proceeds cleavage to give the desired α -amino acid.
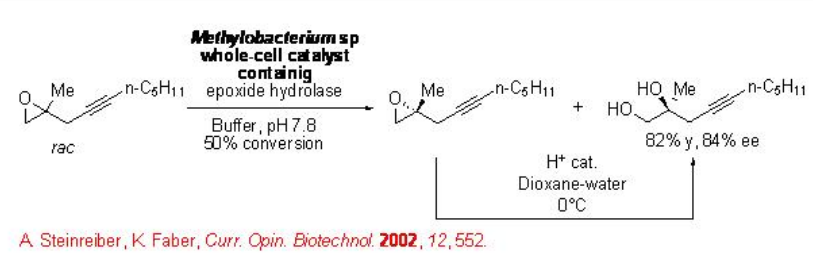
11.2.4 Epoxide Hydrolysis
Hydrolysis of racemic epoxide using epoxide hydrolase proceeds with high enantioselectivity. For example, the resolution of aliphatic epoxide having functional group can be accomplished using Methylobacterium sp. with good enantioselectivity (Scheme \(\PageIndex{13}\)).