10.1: Chiral Proline Based Reactions
- Page ID
- 168829
Enantioselective organocatalysis has emerged as a powerful synthetic method complementary to the metal- and enzyme-catalyzed reactions. The low toxicity associated with organocatalysis and operational simplicity makes it an attractive method to synthesize complex structures. Among the organocatalysts, small molecules like chiral proline, chiral thiourea, chiral TADDOL and chiral alkaloids have special reactivity in the asymmetric synthesis.
Chiral proline is termed as the simplest bifunctional organocatalysts (Scheme \(\PageIndex{1}\)). This amino acid is called as “simplest enzyme” due to its ability to catalyze reactions with high stereoselectivity.
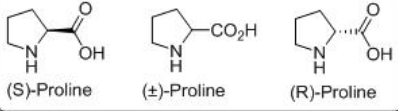
L-Proline is a small molecule, non-toxic, inexpensive, readily available in both enantiomeric forms having bifunctional acid-base sites (Scheme \(\PageIndex{2}\)). The reaction may proceed through either iminium catalysis, or enamine catalysis or bifunctional acid–base catalysis.

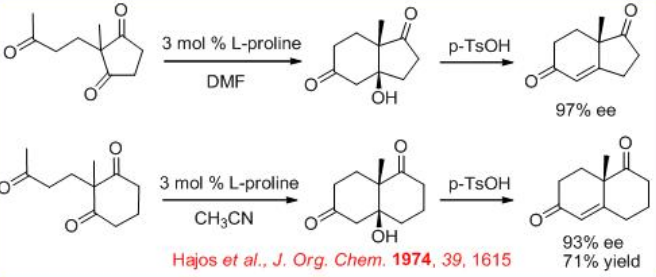
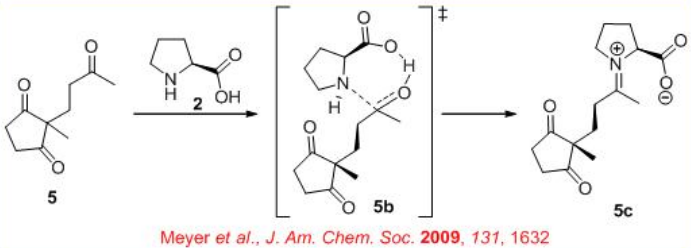
In the early 1970, the first L-proline-catalyzed aldol cyclization was appeared (Scheme \(\PageIndex{3}\)). After nearly 25 years, the expected transition state for the reaction has been illustrated (Scheme \(\PageIndex{4}\)).
Intermolecular Aldol Reaction
The enantioselective aldol reaction is one of the most powerful methods for the construction of chiral polyol. The first intermolecular direct enantioselective aldol reaction catalyzed by L-proline appeared employing acetone and 4-nitrobenzaldehyde as the substrates (Scheme \(\PageIndex{5}\)). This result sparked high interest from several groups in further investigating proline-catalyzed direct asymmetric aldol reactions. Subsequently, modified chiral proline derived catalysts L1-3 has been developed to enhance the selectivity of the reaction.
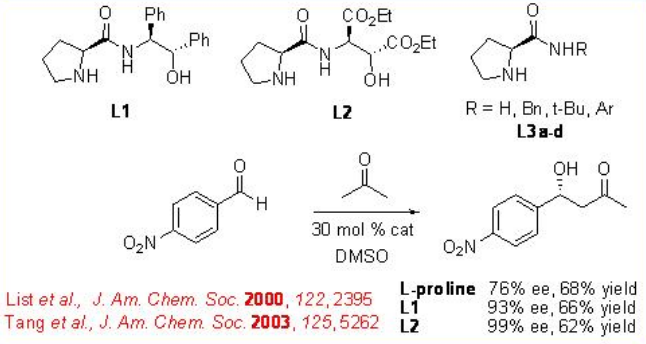
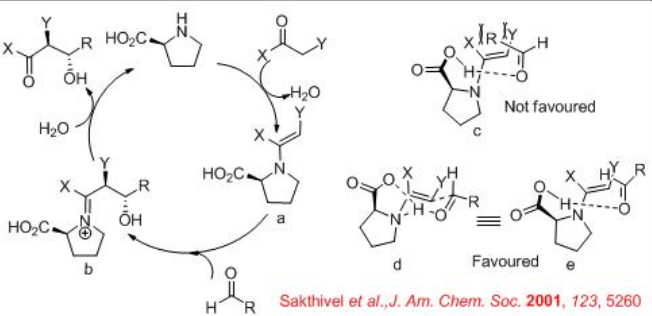
For the mechanism, reaction of pyrrolidine with the carbonyl donor can give enamine a that could proceed reaction with the re -face of the aldehydes to give the iminium ion b (Scheme \(\PageIndex{6}\)). The latter can undergo hydrolysis to afford chiral β-hydroxyketone. The proposed transition state illustrates that enamine attack occurs on the re -face of the aldehyde d and e. This facial selectivity of attack by the enamine is dictated by minimizing steric interactions between the aldehyde substituent and the enamine substituent. The attack of the enamine on the si -face of the aldehyde leads to the unfavorable transition state c .
Mannich Reaction
Parallel to the aldol reaction, enantioselective Mannich reaction of aldehyde, acetone and p -anisidine as the substrates has been explored with 50% yield and 94% ee (Scheme \(\PageIndex{7}\)).
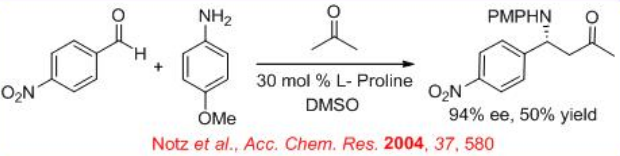
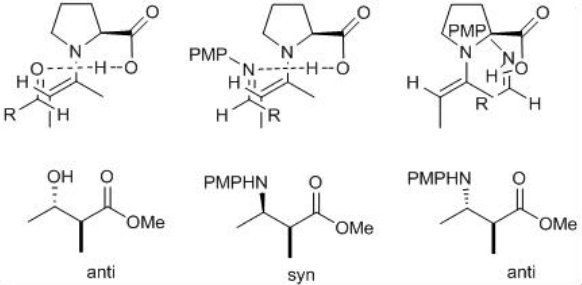
The mechanism is analogous to that of the aldol reactions (Scheme \(\PageIndex{8}\)). The reaction of proline with aldehyde or ketone can give enamine that could undergo reaction with the imine to form new stereocenters as iminium product. The latter on hydrolysis can give the target Mannich product. The reaction of ( E )-aldimine with the enamine on its si -face can give the syn product. Because of the re -face is blocked by steric interactions between the aromatic ring of the p -methoxyphenyl group and the ring of proline.
The proline-catalyzed Mannich reactions of N -PMP-protected α-imino ethyl glyoxylate with a variety of ketones afford functionalized α-amino acids (Scheme 9). These reactions can generate two adjacent stereogenic centers simultaneously upon C-C bond formation with complete syn -stereocontrol and can be performed in a gram scale with operational simplicity.
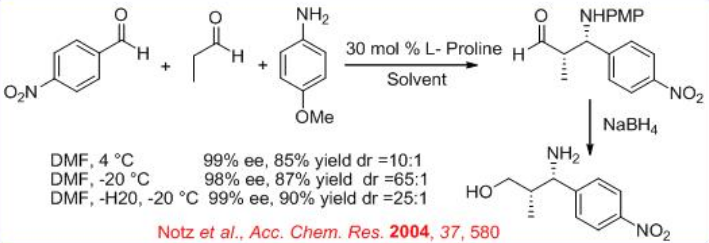
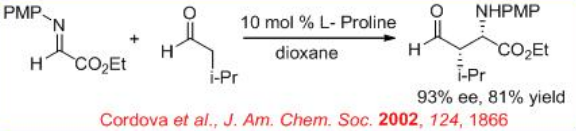
The proline-catalyzed reaction of N -PMP-protected α-imino ethyl glyoxylate with aliphatic aldehydes provides a general method for synthesis of β-amino and α-amino acid derivatives (Scheme \(\PageIndex{10}\)). The diastereoselectivity depends on the bulkiness of the substituents of the aldehyde donor. In most of cases high syn stereoselectivity can be achieved.
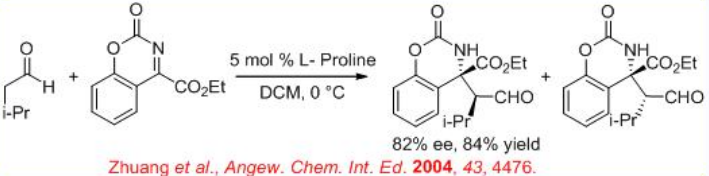
The synthesis of chiral quaternary amino acid derivatives can be accomplished using proline based catalysis (Scheme \(\PageIndex{11}\)). The nitrogen is tethered to the α -aryl amine in order to increase the reactivity through ring strain and the products are obtained with high enantioselectivity.
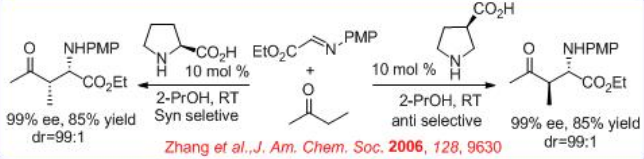
( S )-Proline-catalyzed Mannich-type reaction of aldehydes with α-imino ethyl glyoxylate affords syn -products, while the reaction utilizing (3R, 5R)-5-methyl-3-pyrrolidinecarboxylic acid gives anti -selective product (Scheme \(\PageIndex{12}\)).
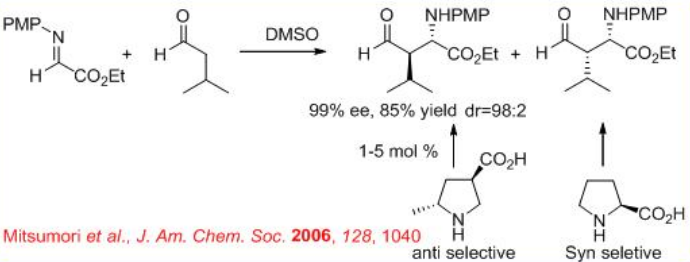
In addition , (R )-3-pyrrolidinecarboxylic acid catalyzes the Mannich-type reactions of ketones with α-imino ethyl glyoxylate to give anti -products, while (S)- proline based reactions give syn -products (Scheme \(\PageIndex{13}\)). Thus, the position of the carboxylic acid group on the pyrrolidine ring directs the stereoselection of the catalyzed reaction providing either syn - or anti -Mannich products.
Michael Reaction
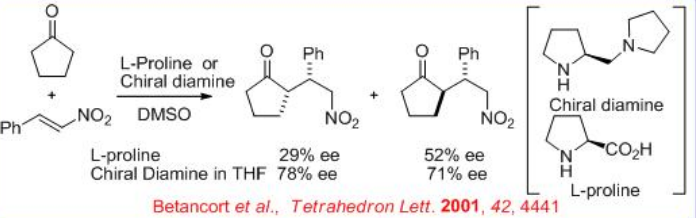
In 2001, the first example for a direct asymmetric Michael reaction employing an enamine-activated donor appeared. The proline-catalyzed reaction of acetone and cyclopentanone with benzalmalonate and nitrostyrene affords the Michael product with low enantiomeric excess. However, the use of chiral diamine improves the ee significantly with both nitrostyrene and alkylidene malonates as acceptors and ketone donors (Scheme \(\PageIndex{14}\)).
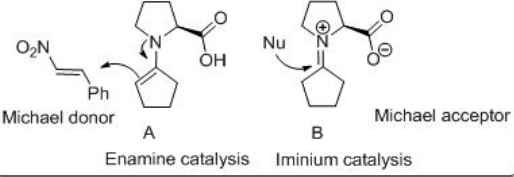
Possible stereochemical result has been accounted by assuming acyclic transition states A and B . These Michael reactions constituted the first direct catalytic asymmetric reactions of any type s involving aldehyde donors and encouraged the development of aldehyde-based reactions with a range of electrophiles (Scheme \(\PageIndex{15}\)).
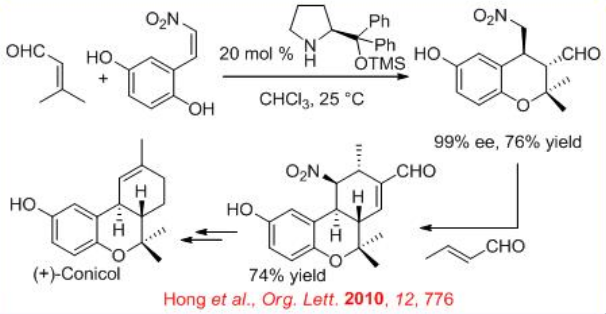
The iminium-enamine activation mode can be envisaged to explain the domino oxa-Michael–Michael reaction occurring between 3-methylbut-2-enal and ( E )-2-(2-nitrovinyl)-benzene-1,4-diol upon catalysis with chiral diphenyl prolinol silyl ether, which afford the corresponding enantiopure oxa-Michael–Michael cycloadduct in 76% yield and 99% ee (Scheme \(\PageIndex{16}\)). The latter can be further implicated in a Michael–aldol sequence through the reaction with crotonaldehyde to afford corresponding hexahydro-6H-benzo-chromene in 74% yield. These two domino reactions have constituted the key steps of the first asymmetric total synthesis of the natural biologically active product (+)-conicol.