29.5: Correlation of Polymer Properties with Structure
- Page ID
- 22396
The properties of many of the commercially important thermoplastic and elastic polymers can be understood in terms of their chemical structures by using the concepts developed in the preceding section. Thus the simple linear polymers, polyethene \(\ce{-(CH_2CH_2)}_n-\), polymethanal \(\ce{-(CH_2-O)}_n-\), and polytetrafluoroethene \(\ce{-(CF_2-CF_2)}_n-\), with regular chains and low barriers to rotation about the bonds in the chain tend to be largely crystalline with rather high melting points and low glass temperatures (see Table 29-1). The situation with polychloroethene (polyvinyl chloride), polyfluoroethene (polyvinyl fluoride), and polyethenylbenzene (polystyrene) as usually prepared is quite different. These polymers are much less crystalline and yet have rather high glass temperatures, which suggests that there is considerable attractive force between the chains. The low degree of crystallinity of these polymers is the result of their having a low degree of regularity of the stereochemical configuration of the chiral carbons in the chain. The discovery by G. Natta in 1954 that the stereochemical configurations of chiral centers in polymer chains could be crucial in determining their physical properties has had a profound impact on both the practical and theoretical aspects of polymer chemistry. Natta's work was done primarily with polypropene and this substance provides an excellent example of the importance of stereochemical configurations.
Table 29-1: Representative Synthetic Thermoplastic and Elastic Polymers and Their Uses\(^a\)
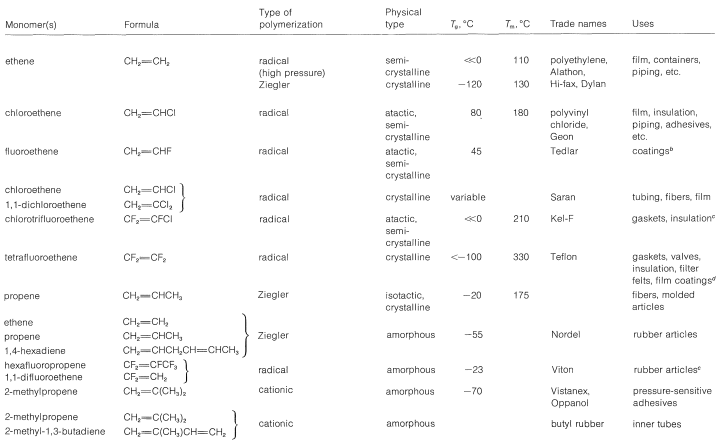
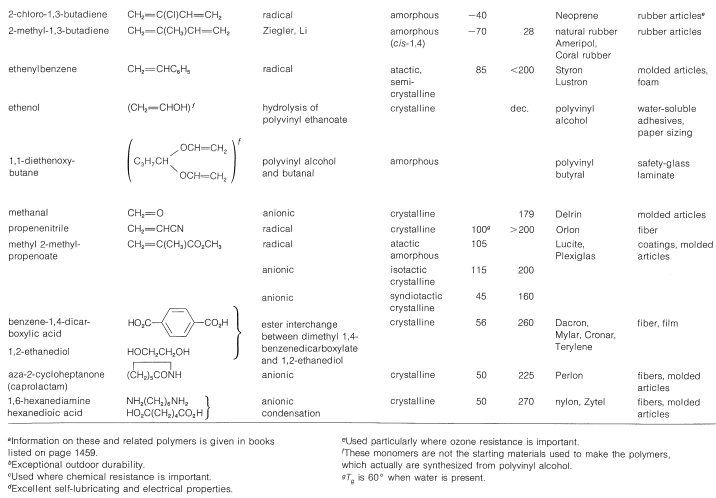
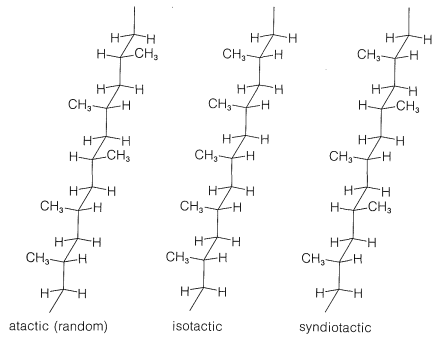
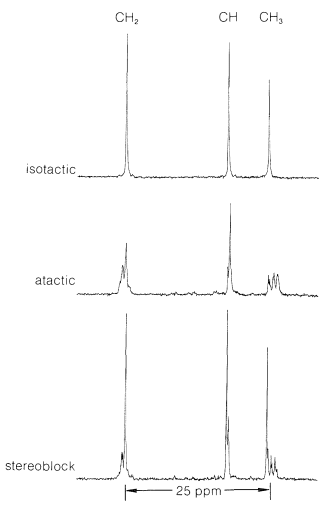
There are striking differences in physical properties between the atactic and isotactic forms. The atactic material is soft, elastic, somewhat sticky, and rather soluble in solvents such as 1,1,2,2-tetrachloroethane. Isotactic polypropene is a hard, clear, strong crystalline polymer that melts at \(175^\text{o}\). It is practically insoluble in all organic solvents at room temperature, but will dissolve to the extent of a few percent in hot 1,1,2,2-tetrachloroethane. That the difference between the atactic and isotactic polymers irises from differences in the configurations of the methyl groups on the chains is shown in a striking way by \(\ce{^{13}C}\) nmr spectra (Figure 29-9). The differences in these spectra result from differences in the interactions between the methyl groups for the different configurations, in the same way as we have shown you earlier for axial and equatorial methyl groups on cyclohexane rings (Section 12-3D).
Why should polypropene melt so much higher than polyethene (\(175^\text{o}\) vs. \(110^\text{o}\))? The answer lies in the differences between the way the polymers crystallize. Polyethene crystallites have extended zig-zag chains that have very low barriers to rotation about the \(\ce{C-C}\) bonds. Because of interferences between the methyl groups, polypropene does not crystallize in extended zig-zag chains but instead forms a helix, something like the \(\alpha\) helix (Section 25-8A), with the chain carbons on the inside and the methyl carbons on the outside. These coils are more rigid than the extended \(\ce{CH_2}\) chains in polyethene and have stabilizing interchain \(\ce{H} \cdots \ce{H}\) interactions so that a higher temperature is required for melting. Polypropene can be cold drawn to form fibers that resemble nylon fibers although, as might be expected, these fibers do not match the \(270^\text{o}\) melting point of nylon and, because of their hydrocarbon character, are much more difficult to dye.
Although both linear polyethene and isotactic polypropene are crystalline polymers, ethene-propene copolymers prepared with the aid of Ziegler catalysts are excellent elastomers. Apparently, a more or less random introduction of methyl groups along a polyethene chain reduces the crystallinity sufficiently drastically to lead to an amorphous polymer. The ethene-propene copolymer is an inexpensive elastomer, but having no double bonds, is not capable of vulcanization. Polymerization of ethene and propene in the presence of a small amount of dicyclopentadiene or 1,4-hexadiene gives an unsaturated heteropolymer, which can be vulcanized with sulfur in the usual way.
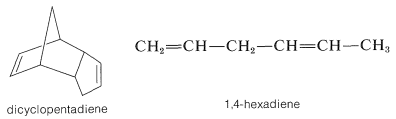
The rationale in using these particular dienes is that only the strained double bond of dicyclopentadiene and the terminal double bond of 1,4-hexadiene undergo polymerization with Ziegler catalysts. Consequently the polymer chains contain one double bond for each molecule of dicyclopentadiene or 1,4-hexadiene that is incorporated. These double bonds later can be converted to cross-links by vulcanization with sulfur (Sections 13-4 and 29-3).
Polychloroethene (polyvinyl chloride), as usually prepared, is atactic and not very crystalline. It is relatively brittle and glassy. The properties of polyvinyl chloride can be improved by copolymerization, as with ethenyl ethanoate (vinyl acetate), which produces a softer polymer ("Vinylite") with better molding properties. Polyvinyl chloride also can be plasticized by blending it with substances of low volatility such as tris-(2-methylphenyl) phosphate (tricresyl phosphate) and dibutyl benzene-1,2-dicarboxylate (dibutyl phthalate) which, when dissolved in the polymer, tend to break down its glasslike structure. Plasticized polyvinyl chloride is reasonably flexible and is widely used as electrical insulation, plastic sheeting, and so on.
Table 29-1 contains information about a number of representative important polymers and their uses. Some similar data on other polymers already have been given (Section 13-4 and Table 10-4). The important use of modified polymers as ion-exchange resins is discussed in Section 25-4C.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


